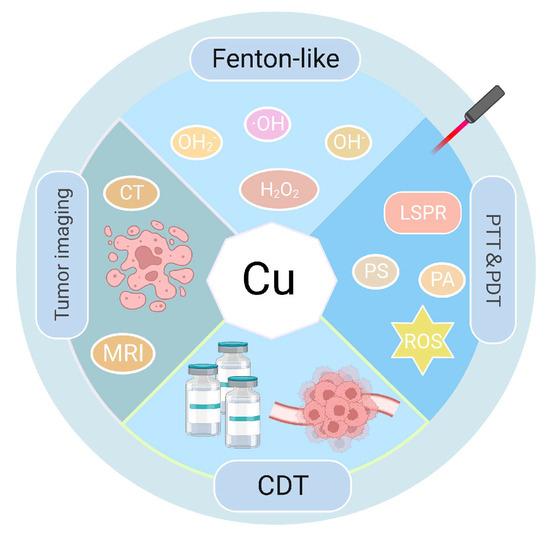
纳米技术是一种新兴且有前途的治疗工具,由于具有吸光特性的纳米材料(Nanotechnology, an emerging and promising therapeutic tool, may improve the effectiveness of phototherapy (PT) in antitumor therapy because of the development of nanomaterials (NMs)的发展,可能会提高光疗(PT)在抗肿瘤治疗中的有效性。肿瘤靶向PTs,如光热疗法(PTT)和光动力疗法(PDT),将光能转化为热量并产生积聚在肿瘤部位的活性氧(ROS)。ROS水平的增加在致癌和疾病发展过程中诱导氧化应激(OS)。由于铜(Cu)是人体中重要的微量元素,具有局部表面等离子体共振(LSPR)特性,Cu基NMs可以表现出良好的近红外(NIR)吸收和优异的光热性能。与其他治疗方式相比,) with light-absorbing properties. The tumor-targeted PTs, such as photothermal therapy (PTT) and photodynamic therapy (PDT), transform light energy into heat and produce reactive oxygen species (ROS) that accumulate at the tumor site. The increase in ROS levels induces oxidative stress (OS) during carcinogenesis and disease development. Because of the localized surface plasmon resonance (LSPR) feature of copper (Cu), a vital trace element in the human body, Cu-based NMs can exhibit good near-infrared (NIR) absorption and excellent photothermal properties. Compared with other therapeutic modalities, PTT/PDT可以精确地靶向肿瘤位置以杀死肿瘤细胞。此外,多种治疗方式可以与PTT / PDT结合使用,以使用基于Cu的NM治疗肿瘤。 can precisely target tumor location to kill tumor cells. Moreover, multiple treatment modalities can be combined with PTT/PDT to treat a tumor using Cu-based NMs.
- nanomaterials (NMs)
- photodynamic therapy (PDT)
- photothermal therapy (PTT)
- copper (Cu)
- Cu-based NMs
- reactive oxygen species (ROS)
1. 简介Introduction
2. 氧化铜在Application of Copper Oxides in PTT和 and PDT中的应用
3. 铜的应用Application of CuxSy在 in PTT 和and PDT 中
4. Application of Copper Selenides and Copper Telluride in PTT and PDT
5. Application of Cu-Based Nanocomposites in PTT and PDT
6. Application of Cu-MOF in PTT and PDT
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Song, G.; Chen, Y.; Liang, C.; Yi, X.; Liu, J.; Sun, X.; Shen, S.; Yang, K.; Liu, Z. Catalase-Loaded TaOx Nanoshells as Bio-Nanoreactors Combining High-Z Element and Enzyme Delivery for Enhancing Radiotherapy. Adv. Mater. 2016, 28, 7143–7148.
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565.
- Arroyo-Hernández, M.; Maldonado, F.; Lozano-Ruiz, F.; Muñoz-Montaño, W.; Nuñez-Baez, M.; Arrieta, O. Radiation-induced lung injury: Current evidence. BMC Pulm. Med. 2021, 21, 9.
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726.
- Chari, R.V.; Miller, M.L.; Widdison, W.C. Antibody-drug conjugates: An emerging concept in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 3796–3827.
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug. Deliv. Rev. 2020, 163, 125–144.
- Gulzar, A.; Wang, Z.; He, F.; Yang, D.; Zhang, F.; Gai, S.; Yang, P. An 808 nm Light-Sensitized Upconversion Nanoplatform for Multimodal Imaging and Efficient Cancer Therapy. Inorg. Chem. 2020, 59, 4909–4923.
- Zhang, L.X.; Sun, X.M.; Xu, Z.P.; Liu, R.T. Development of Multifunctional Clay-Based Nanomedicine for Elimination of Primary Invasive Breast Cancer and Prevention of Its Lung Metastasis and Distant Inoculation. ACS Appl. Mater. Interfaces 2019, 11, 35566–35576.
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838.
- Li, Y.; Wen, T.; Zhao, R.; Liu, X.; Ji, T.; Wang, H.; Shi, X.; Shi, J.; Wei, J.; Zhao, Y.; et al. Localized Electric Field of Plasmonic Nanoplatform Enhanced Photodynam ic Tumor Therapy. ACS Nano 2014, 8, 11529–11542.
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018, 163, 14–24.
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11.
- Sobis, H.; Waer, M.; Vandeputte, M. Normal and malignant trophoblasts do not recruit granulated metrial gland cells. Tumour Biol. 1996, 17, 13–19.
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108.
- Cai, X.; Gao, W.; Zhang, L.; Ma, M.; Liu, T.; Du, W.; Zheng, Y.; Chen, H.; Shi, J. Enabling Prussian Blue with Tunable Localized Surface Plasmon Resonanc es: Simultaneously Enhanced Dual-Mode Imaging and Tumor Photothermal T herapy. ACS Nano 2016, 10, 11115–11126.
- Jin, T.; Cheng, D.; Jiang, G.; Xing, W.; Liu, P.; Wang, B.; Zhu, W.; Sun, H.; Sun, Z.; Xu, Y.; et al. Engineering naphthalimide-cyanine integrated near-infrared dye into ROS-responsive nanohybrids for tumor PDT/PTT/chemotherapy. Bioact. Mater. 2022, 14, 42–51.
- Zanganeh, N.; Guda, V.K.; Toghiani, H.; Keith, J.M. Sinter-Resistant and Highly Active Sub-5 nm Bimetallic Au–Cu Nanoparticle Catalysts Encapsulated in Silica for High-Temperature Carbon Monoxide Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 4776–4785.
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85.
- Giodini, L.; Re, F.L.; Campagnol, D.; Marangon, E.; Posocco, B.; Dreussi, E.; Toffoli, G. Nanocarriers in cancer clinical practice: A pharmacokinetic issue. Nanomedicine 2017, 13, 583–599.
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7.
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Semin. Cancer Biol. 2021, 69, 52–68.
- Osaki, T.; Yokoe, I.; Sunden, Y.; Ota, U.; Ichikawa, T.; Imazato, H.; Ishii, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; et al. Efficacy of 5-Aminolevulinic Acid in Photodynamic Detection and Photodynamic Therapy in Veterinary Medicine. Cancers 2019, 11, 495.
- Gao, W.; Wang, Z.; Lv, L.; Yin, D.; Chen, D.; Han, Z.; Ma, Y.; Zhang, M.; Yang, M.; Gu, Y. Photodynamic Therapy Induced Enhancement of Tumor Vasculature Permeability Using an Upconversion Nanoconstruct for Improved Intratumoral Nanoparticle Delivery in Deep Tissues. Theranostics 2016, 6, 1131–1144.
- Xu, M.; Zhou, L.; Zheng, L.; Zhou, Q.; Liu, K.; Mao, Y.; Song, S. Sonodynamic therapy-derived multimodal synergistic cancer therapy. Cancer Lett. 2021, 497, 229–242.
- Lee, H.E.; Ahn, H.Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365.
- Jian, C.-C.; Zhang, J.; Ma, X. Cu–Ag alloy for engineering properties and applications based on the LSPR of metal nanoparticles. RSC Adv. 2020, 10, 13277–13285.
- Gezgin, S.Y.; Kepceoğlu, A.; Gündoğdu, Y.; Zongo, S.; Zawadzka, A.; Kiliç, H.; Sahraoui, B. Effect of Ar Gas Pressure on LSPR Property of Au Nanoparticles: Comparison of Experimental and Theoretical Studies. Nanomaterials 2020, 10, 1071.
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195.
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H2O2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166.
- Al Kayal, T.; Giuntoli, G.; Cavallo, A.; Pisani, A.; Mazzetti, P.; Fonnesu, R.; Rosellini, A.; Pistello, M.; D’Acunto, M.; Soldani, G.; et al. Incorporation of Copper Nanoparticles on Electrospun Polyurethane Memb rane Fibers by a Spray Method. Molecules 2023, 28, 5981.
- Zhang, W.X.; Hao, Y.N.; Gao, Y.R.; Shu, Y.; Wang, J.H. Mutual Benefit between Cu(II) and Polydopamine for Improving Photothermal-Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 38127–38137.
- Tai, Y.W.; Chiu, Y.C.; Wu, P.T.; Yu, J.; Chin, Y.C.; Wu, S.P.; Chuang, Y.C.; Hsieh, H.C.; Lai, P.S.; Yu, H.P.; et al. Degradable NIR-PTT Nanoagents with a Potential Cu@Cu2O@Polymer Structure. ACS Appl. Mater. Interfaces 2018, 10, 5161–5174.
- Yuan, H.; Xia, P.; Sun, X.; Ma, J.; Xu, X.; Fu, C.; Zhou, H.; Guan, Y.; Li, Z.; Zhao, S.; et al. Photothermal Nanozymatic Nanoparticles Induce Ferroptosis and Apoptosis through Tumor Microenvironment Manipulation for Cancer Therapy. Small 2022, 18, e2202161.
- Xu, N.; Hu, A.; Pu, X.; Wang, J.; Liao, X.; Huang, Z.; Yin, G. Cu-Chelated polydopamine nanoparticles as a photothermal medium and “immunogenic cell death” inducer for combined tumor therapy. J. Mater. Chem. B 2022, 10, 3104–3118.
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199.
- Aishajiang, R.; Liu, Z.; Wang, T.; Zhou, L.; Yu, D. Recent Advances in Cancer Therapeutic Copper-Based Nanomaterials for Antitumor Therapy. Molecules 2023, 28, 2303.
- Xiang, H.; Xue, F.; Yi, T.; Tham, H.P.; Liu, J.-G.; Zhao, Y. Cu2-xS Nanocrystals Cross-Linked with Chlorin e6-Functiona lized Polyethylenimine for Synergistic Photodynamic and Photothermal T herapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 16344–16351.
- Zhong, X.; Dai, X.; Wang, Y.; Wang, H.; Qian, H.; Wang, X. Copper-based nanomaterials for cancer theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1797.
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily b y targeting tumor initiating cells. Sci. Rep. 2019, 9, 12613.
- Liu, Y.; Zhen, W.; Jin, L.; Zhang, S.; Sun, G.; Zhang, T.; Xu, X.; Song, S.; Wang, Y.; Liu, J.; et al. All-in-One Theranostic Nanoagent with Enhanced Reactive Oxygen Species Generation and Modulating Tumor Microenvironment Ability for Effective Tumor Eradication. ACS Nano 2018, 12, 4886–4893.
- Rehman, S.U.; Zubair, H.; Sarwar, T.; Husain, M.A.; Ishqi, H.M.; Nehar, S.; Tabish, M. Redox cycling of Cu(II) by 6-mercaptopurine leads to ROS generation and DNA breakage: Possible mechanism of anticancer activity. Tumour Biol. 2015, 36, 1237–1244.
- Jiang, F.; Ding, B.; Zhao, Y.; Liang, S.; Cheng, Z.; Xing, B.; Teng, B.; Ma, P.A.; Lin, J. Biocompatible CuO-decorated carbon nanoplatforms for multiplexed imaging and enhanced antitumor efficacy via combined photothermal therapy/chemodynamic therapy/chemotherapy. Sci. China Mater. 2020, 63, 1818–1830.
- Jiang, F.; Ding, B.; Liang, S.; Zhao, Y.; Cheng, Z.; Xing, B.; Ma, P.A.; Lin, J. Intelligent MoS2–CuO heterostructures with multiplexed imaging and remarkably enhanced antitumor efficacy via synergetic photothermal therapy/chemodynamic therapy/ immunotherapy. Biomaterials 2021, 268, 120545.
- Palashuddin Sk, M.; Goswami, U.; Ghosh, S.S.; Chattopadhyay, A. Cu2+-embedded carbon nanoparticles as anticancer agents. J. Mater. Chem. B 2015, 3, 5673–5677.
- Yuan, R.; Xu, H.; Liu, X.; Tian, Y.; Li, C.; Chen, X.; Su, S.; Perelshtein, I.; Gedanken, A.; Lin, X. Zinc-Doped Copper Oxide Nanocomposites Inhibit the Growth of Human Cancer Cells through Reactive Oxygen Species-Mediated NF-κB Activations. ACS Appl. Mater. Interfaces 2016, 8, 31806–31812.
- Han, L.; Zhang, Y.; Chen, X.W.; Shu, Y.; Wang, J.H. Protein-modified hollow copper sulfide nanoparticles carrying indocyanine green for photothermal and photodynamic therapy. J. Mater. Chem. B 2016, 4, 105–112.
- Chen, G.; Ma, B.; Wang, Y.; Xie, R.; Li, C.; Dou, K.; Gong, S. CuS-Based Theranostic Micelles for NIR-Controlled Combination Chemotherapy and Photothermal Therapy and Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2017, 9, 41700–41711.
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 nanocrystals: A photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 2011, 5, 9761–9771.
- Tian, Q.; Tang, M.; Sun, Y.; Zou, R.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 2011, 23, 3542–3547.
- Li, B.; Jiang, Z.; Xie, D.; Wang, Y.; Lao, X. Cetuximab-modified CuS nanoparticles integrating near-infrared-II-responsive photothermal therapy and anti-vessel treatment. Int. J. Nanomed. 2018, 13, 7289–7302.
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129.
- Li, G.; Yuan, S.; Deng, D.; Ou, T.; Li, Y.; Sun, R.; Lei, Q.; Wang, X.; Shen, W.; Cheng, Y.; et al. Fluorinated Polyethylenimine to Enable Transmucosal Delivery of Photosensitizer-Conjugated Catalase for Photodynamic Therapy of Orthotopic Bladder Tumors Postintravesical Instillation. Adv. Funct. Mater. 2019, 29, 1901932.
- Mu, X.; Chang, Y.; Bao, Y.; Cui, A.; Zhong, X.; Cooper, G.B.; Guo, A.; Shan, G. Core-satellite nanoreactors based on cationic photosensitizer modified hollow CuS nanocage for ROS diffusion enhanced phototherapy of hypoxic tumor. Biomater. Adv. 2023, 145, 213263.
- Feng, L.; Zhao, R.; Liu, B.; He, F.; Gai, S.; Chen, Y.; Yang, P. Near-Infrared Upconversion Mesoporous Tin Oxide Bio-Photocatalyst for H2O2Activatable O2-Generating Magnet ic Targeting Synergetic Treatment. ACS Appl. Mater. Interfaces 2020, 12, 41047–41061.
- Akbari, M.; Sogutdelen, E.; Juriasingani, S.; Sener, A. Hydrogen Sulfide: Emerging Role in Bladder, Kidney, and Prostate Malignancies. Oxid. Med. Cell. Longev. 2019, 2019, 2360945.
- Feng, L.; Wu, S.; Wu, Y. Intracellular Bottom-up Synthesis of Ultrasmall CuS Nanodots in Cancer Cells for Simultaneous Photothermal Therapy and COX-2 Inactivation. Adv. Funct. Mater. 2021, 31, 2101297.
- Shi, H.; Sun, Y.; Yan, R.; Liu, S.; Zhu, L.; Liu, S.; Feng, Y.; Wang, P.; He, J.; Zhou, Z.; et al. Magnetic Semiconductor Gd-Doping CuS Nanoparticles as Activatable Nanoprobes for Bimodal Imaging and Targeted Photothermal Therapy of Gastric Tumors. Nano Lett. 2019, 19, 937–947.
- Zhao, R.; Sun, X.; Sun, J.; Wang, L.; Han, J. Polypyrrole-modified CuS nanoprisms for efficient near-infrared photothermal therapy. RSC Adv. 2017, 7, 10143–10149.
- Liu, X.; Law, W.-C.; Jeon, M.; Wang, X.; Liu, M.; Kim, C.; Prasad, P.N.; Swihart, M.T. Cu2-x Se nanocrystals with localized surface plasmon resonance as sens itive contrast agents for in vivo photoacoustic imaging: Demonstration of sentinel lymph node mapping. Adv. Healthc. Mater. 2013, 2, 952–957.
- Zhang, S.; Sun, C.; Zeng, J.; Sun, Q.; Wang, G.; Wang, Y.; Wu, Y.; Dou, S.; Gao, M.; Li, Z. Ambient Aqueous Synthesis of Ultrasmall PEGylated Cu2xSe Nanoparticles as a Multifunctional Theranostic Agent for Multimodal Imaging Guided Photothermal Therapy of Cancer. Adv. Mater. 2016, 28, 8927–8936.
- Zeng, J.; Goldfeld, D.; Xia, Y. A plasmon-assisted optofluidic (PAOF) system for measuring the phototh ermal conversion efficiencies of gold nanostructures and controlling a n electrical switch. Angew. Chem. 2013, 52, 4169–4173.
- Wang, Y.; Li, Z.; Hu, Y.; Liu, J.; Guo, M.; Wei, H.; Zheng, S.; Jiang, T.; Sun, X.; Ma, Z.; et al. Photothermal conversion-coordinated Fenton-like and photocatalytic rea ctions of Cu2-xSe-Au Janus nanoparticles for tri-combinatio n antitumor therapy. Biomaterials 2020, 255, 120167.
- Yan, H.; Dong, J.; Luan, X.; Wang, C.; Song, Z.; Chen, Q.; Ma, J.; Du, X. Ultrathin Porous Nitrogen-Doped Carbon-Coated CuSe Heterostructures fo r Combination Cancer Therapy of Photothermal Therapy, Photocatalytic T herapy, and Logic-Gated Chemotherapy. ACS Appl. Mater. Interfaces 2022, 14, 56237–56252.
- Gao, L.; Chen, Q.; Gong, T.; Liu, J.; Li, C. Recent advancement of imidazolate framework (ZIF-8) based nanoformulat ions for synergistic tumor therapy. Nanoscale 2019, 11, 21030–21045.
- Liu, T.; Xu, L.; He, L.; Zhao, J.; Zhang, Z.; Chen, Q.; Chen, T. Selenium nanoparticles regulates selenoprotein to boost cytokine-induc ed killer cells-based cancer immunotherapy. Nano Today 2020, 35, 100975.
- Zou, B.; Xiong, Z.; He, L.; Chen, T. Reversing breast cancer bone metastasis by metal organic framework-cap ped nanotherapeutics via suppressing osteoclastogenesis. Biomaterials 2022, 285, 121549.
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566.
- Kumar, P.; Singh, K.; Srivastava, O.N. Template free-solvothermaly synthesized copper selenide (CuSe, Cu2−xSe, β-Cu2Se and Cu2Se) hexagonal nanoplates from different precursors at low temperature. J. Cryst. Growth 2010, 312, 2804–2813.
- Hu, C.; Zhang, Z.; Liu, S.; Liu, X.; Pang, M. Monodispersed CuSe Sensitized Covalent Organic Framework Photosensitizer with an Enhanced Photodynamic and Photothermal Effect for Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23072–23082.
- Li, W.; Zamani, R.; Rivera Gil, P.; Pelaz, B.; Ibáñez, M.; Cadavid, D.; Shavel, A.; Alvarez-Puebla, R.A.; Parak, W.J.; Arbiol, J.; et al. CuTe Nanocrystals: Shape and Size Control, Plasmonic Properties, and U se as SERS Probes and Photothermal Agents. J. Am. Chem. Soc. 2013, 135, 7098–7101.
- Zhou, G.; Li, M. Biodegradable copper telluride nanosheets for redox-homeostasis breaki ng-assisted chemodynamic cancer therapy boosted by mild-photothermal e ffect. Chem. Eng. J. 2022, 450, 138348.
- Fang, T.; Ma, S.; Wei, Y.; Yang, J.; Zhang, J.; Shen, Q. Catalytic immunotherapy-photothermal therapy combination for melanoma by ferroptosis-activating vaccine based on artificial nanoenzyme. Mater. Today Chem. 2023, 27, 101308.
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. Buon. 2018, 23, 561–567.
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261.
- Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472.
- Xu, Y.; Liu, S.Y.; Zeng, L.; Ma, H.; Zhang, Y.; Yang, H.; Liu, Y.; Fang, S.; Zhao, J.; Xu, Y.; et al. An Enzyme-Engineered Nonporous Copper(I) Coordination Polymer Nanoplatform for Cuproptosis-Based Synergistic Cancer Therapy. Adv. Mater. 2022, 34, e2204733.
- Zhang, H.; Liu, K.; Li, S.; Xin, X.; Yuan, S.; Ma, G.; Yan, X. Self-Assembled Minimalist Multifunctional Theranostic Nanoplatform for Magnetic Resonance Imaging-Guided Tumor Photodynamic Therapy. ACS Nano 2018, 12, 8266–8276.
- Wang, X.-S.; Zeng, J.-Y.; Zhang, M.-K.; Zeng, X.; Zhang, X.-Z. A Versatile Pt-Based Core-Shell Nanoplatform as a Nanofactory for Enha nced Tumor Therapy. Adv. Funct. Mater. 2018, 28, 1801783.
- Chen, Z.; Wu, Y.; Yao, Z.; Su, J.; Wang, Z.; Xia, H.; Liu, S. 2D Copper(II) Metalated Metal-Organic Framework Nanocomplexes for Dual-enhanced Photodynamic Therapy and Amplified Antitumor Immunity. ACS Appl. Mater. Interfaces 2022, 14, 44199–44210.
- Huang, Z.; Chen, Y.; Zhang, J.; Li, W.; Shi, M.; Qiao, M.; Zhao, X.; Hu, H.; Chen, D. Laser/GSH-Activatable Oxaliplatin/Phthalocyanine-Based Coordination Polymer Nanoparticles Combining Chemophotodynamic Therapy to Improve Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 39934–39948.
- Yan, L.; Gonca, S.; Zhu, G.; Zhang, W.; Chen, X. Layered double hydroxide nanostructures and nanocomposites for biomedical applications. J. Mater. Chem. B 2019, 7, 5583–5601.
- Sun, L.; Wang, J.; Liu, J.; Li, L.; Xu, Z.P. Creating Structural Defects of Drug-Free Copper-Containing Layered Double Hydroxide Nanoparticles to Synergize Photothermal/Photodynamic/Chemodynamic Cancer Therapy. Small Struct. 2021, 2, 2000112.
- Zhang, J.; Mukamel, S.; Jiang, J. Aggregation-Induced Intersystem Crossing: Rational Design for Phosphorescence Manipulation. J. Phys. Chem. B 2020, 124, 2238–2244.
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-Activatable and O2-Evolving Nanoparticles for Highly Efficient and Selective Photodynamic Therapy against Hypoxic Tumor Cells. J. Am. Chem. Soc. 2015, 137, 1539–1547.
- Yang, J.; Wang, Y.; Qin, G.; Tian, T.; Ran, J.; Wang, H.; Yang, C. Photogeneration of Hydroxyl Radicals Based on Aggregation-Induced Emission Luminogen-Assembled Copper Cysteamine Nanoparticles for Photodynamic Therapy. ACS Appl. Nano Mater. 2023, 6, 533–543.
- Skrott, Z.; Majera, D.; Gursky, J.; Buchtova, T.; Hajduch, M.; Mistrik, M.; Bartek, J. Disulfiram’s anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase. Oncogene 2019, 38, 6711–6722.
- Wu, W.; Yu, L.; Jiang, Q.; Huo, M.; Lin, H.; Wang, L.; Chen, Y.; Shi, J. Enhanced Tumor-Specific Disulfiram Chemotherapy by In Situ Cu2+ Chelation-Initiated Nontoxicity-to-Toxicity Transition. J. Am. Chem. Soc. 2019, 141, 11531–11539.
- Zhou, J.; Yu, Q.; Song, J.; Li, S.; Li, X.L.; Kang, B.K.; Chen, H.Y.; Xu, J.J. Photothermally Triggered Copper Payload Release for Cuproptosis-Promoted Cancer Synergistic Therapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202213922.
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Membranes. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359.
- Wang, D.; Zhou, J.; Shi, R.; Wu, H.; Chen, R.; Duan, B.; Xia, G.; Xu, P.; Wang, H.; Zhou, S.; et al. Biodegradable Core-shell Dual-Metal-Organic-Frameworks Nanotheranostic Agent for Multiple Imaging Guided Combination Cancer Therapy. Theranostics 2017, 7, 4605–4617.
- Wang, S.; Shang, L.; Li, L.; Yu, Y.; Chi, C.; Wang, K.; Zhang, J.; Shi, R.; Shen, H.; Waterhouse, G.I.; et al. Metal-Organic-Framework-Derived Mesoporous Carbon Nanospheres Containing Porphyrin-Like Metal Centers for Conformal Phototherapy. Adv. Mater. 2016, 28, 8379–8387.
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.C. Size-Controlled Synthesis of Porphyrinic Metal-Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525.
- Li, B.; Ye, K.; Zhang, Y.; Qin, J.; Zou, R.; Xu, K.; Huang, X.; Xiao, Z.; Zhang, W.; Lu, X.; et al. Photothermal theragnosis synergistic therapy based on bimetal sulphide nanocrystals rather than nanocomposites. Adv. Mater. 2015, 27, 1339–1345.
- Li, B.; Wang, X.; Chen, L.; Zhou, Y.; Dang, W.; Chang, J.; Wu, C. Ultrathin Cu-TCPP MOF nanosheets: A new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics 2018, 8, 4086–4096.
- Ju, E.; Dong, K.; Chen, Z.; Liu, Z.; Liu, C.; Huang, Y.; Wang, Z.; Pu, F.; Ren, J.; Qu, X. Copper(II)-Graphitic Carbon Nitride Triggered Synergy: Improved ROS Generation and Reduced Glutathione Levels for Enhanced Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2016, 55, 11467–11471.
- Zhang, W.; Lu, J.; Gao, X.; Li, P.; Zhang, W.; Ma, Y.; Wang, H.; Tang, B. Enhanced Photodynamic Therapy by Reduced Levels of Intracellular Glutathione Obtained By Employing a Nano-MOF with Cu(II) as the Active Center. Angew. Chem. Int. Ed. Engl. 2018, 57, 4891–4896.
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A water-stable porphyrin-based metal-organic framework active for visible-light photocatalysis. Angew. Chem. Int. Ed. Engl. 2012, 51, 7440–7444.
