Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Peter Tang.
Solid oxide fuel cells (SOFCs) represent a breed of eco-friendly, weather-independent, decentralized power generation technologies, distinguished for their broad fuel versatility and superior electricity generation efficiency.
- woshihxd117
- solid oxide fuel cells
- double perovskite
- oxygen
1. Physicochemical Properties of LnBaCo2O5+δ
As depicted in Figure 1, the LnBaCo2O5+δ compound exhibits a perovskite structure of the 112 type. Relative to their disordered analogs, these orderly structures have been widely reported to considerably enhance the rate of oxygen transport [45,46][1][2]. Notably, Taskin et al. [45][1] were pioneers in observing a notably high oxygen diffusion coefficient (Dchem) of approximately 3.0 × 10−⁹ cm² s−¹ at 350 °C and 10−⁵ cm² s−¹ at 600 °C for the GdBaCo2O5+δ double perovskite. The oxygen transport characteristics of the PrBaCo2O5+δ double perovskite were subsequently evaluated by Kim et al. [47,48][3][4]. Their results demonstrated appreciably higher rates of oxygen transport (Dchem) for PrBaCo2O5+δ in comparison to GdBaCo2O5+δ, suggesting an enhancement in oxygen transport properties corresponding to the increased size of the Ln cation. Tarancón et al. carried out a detailed comparative study between the double perovskite LnBaCo2O5+δ (Ln = Pr, Gd) and other classes of oxygen catalysts [44][5]. The double perovskite outperformed in terms of oxygen transport properties, emphasizing its considerable potential as a cutting-edge cathode material for SOFCs. It is important to recognize that significant variations exist in the LnBaCo2O5+δ oxygen tracer diffusion and the oxygen surface exchange coefficient as reported by different research groups [49][6]. Such disparities mainly stem from differences in the precise composition and/or microstructure of the samples used by distinct researchers.
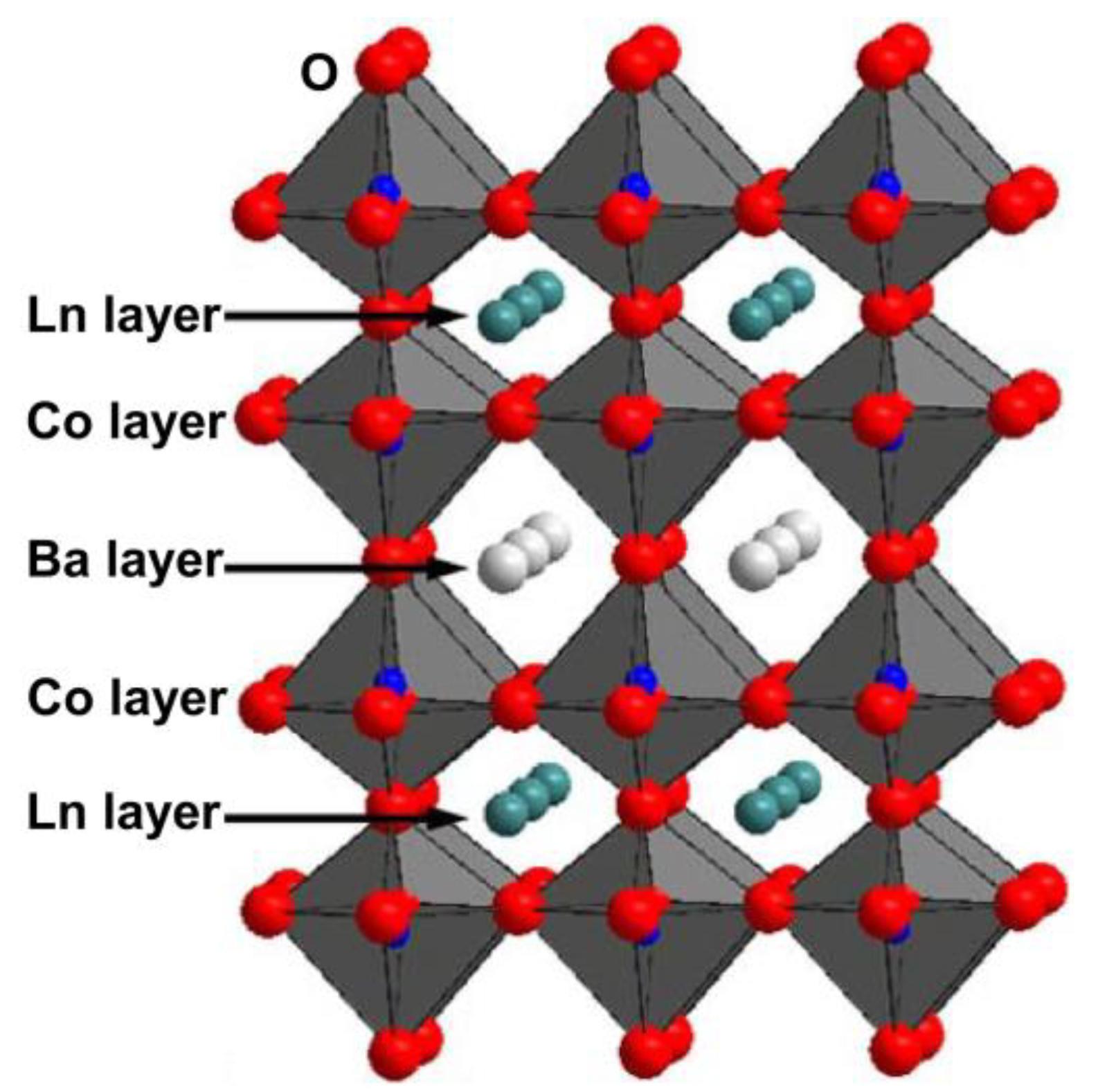
Figure 1.
Schematic diagram of crystal structure for double perovskite oxide LnBaCo
2
O
5+δ.
Numerous experimental and theoretical studies have been undertaken to delve deeper into the oxygen diffusion behaviors in double perovskites [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25]. Seymour et al. utilized static atomistic simulations based on the Born model to methodically examine the intrinsic defect processes of the double perovskite LnBaCo2O5.5 (Ln = Y, La, Pr, Nd, Sm, Gd, Dy, Ho, Er, Yb) [53][7]. Their research indicated that the defect reaction with the lowest energy stemmed from the Ln/Ba antisite disorder energy, which diminishes with decreasing Ln size. This suggests that the ordered structure’s primary foundation is the size difference between the Ln and Ba cations [53][7]. Parfitt et al. combined molecular dynamics with Born model potentials to study the oxygen transport behavior of GdBaCo2O5+δ at 900 K [54,55][8][9]. They posited that A-site cation ordering, in contrast to its disordered equivalent, can amplify oxygen bulk diffusivity while decreasing transport in the c-axis direction [54,55][8][9]. Importantly, the distinctively anisotropic oxygen diffusion in the double perovskite GdBaCo2O5+δ takes place exclusively within the [GdOδ] and adjacent [CoO2] layers. Shiiba et al. probed the distribution of oxygen vacancies in GdBaCo2O5+δ under various oxygen vacancy concentrations (0 ≤ δ ≤ 1) and temperatures using a fusion of density functional theory and Monte Carlo simulation [57][11]. Their analysis showed that oxygen vacancies, which function as oxygen ion carriers, are restricted to the [GdOδ] and neighboring [CoO2] layers, reinforcing the anisotropic oxygen diffusion mechanism. Seymour et al. performed theoretical investigations on the oxygen transport properties of layered PrBaCo2O5+δ at 650 and 1000 °C, employing the MD method [59,60,61,62][13][14][15][16]. These proposed mechanisms for oxygen conducting were later confirmed experimentally via in situ high-temperature neutron powder diffraction and isotope exchange depth profile methods [59,60,61,62][13][14][15][16]. Additionally, it has been shown that PrBaCo2O5+δ has a lower energy barrier for oxygen diffusion perpendicular to the c-axis compared to Nd, suggesting enhanced oxygen ion diffusivity with larger Ln sizes [53,59][7][13]. Wang et al. detected rapid cobalt redox reactions in epitaxial LaBaCo2O5+δ within a temperature bracket of 260–700 °C, intimately tied to the processes of oxygen release and uptake processes [72][26]. This finding hints at the potential application of these films in SOFC cathodes. Notably, Wang et al. found the cobalt oxidation in the epitaxial thin films to be substantially swifter than the reduction process, denoting a more rapid oxygen uptake compared to the oxygen release [72][26]. Bao et al.’s research further revealed a layer-by-layer oxygen transport mechanism in epitaxial double perovskites, specifically LnBaCo2O5+δ (Ln = Pr, Er), which likely originates in their intrinsic anisotropic oxygen diffusion properties [73][27].
LnBaCo2O5+δ, owing to its exceptionally promising properties, has been extensively studied as a cathode material for SOFCs [34,74,75,76,77,78,79,80,81,82,83][28][29][30][31][32][33][34][35][36][37][38]. Researchers have undertaken thorough studies into the structural performance, thermal expansion behavior, electrical conductivity, and electrochemical performance of these double perovskites. Studies on ions such as La3+, Pr3+, Nd3+, Sm3+, and Gd3+ have shown that these oxides exhibit good chemical compatibility with commonly used electrolytes, including GDC, La0.8Sr0.2Ga0.8Mg0.2O2.8 (LSGM), and samarium oxide-doped ceria (SDC), at temperatures below 1000 °C [74,75,76,84][29][30][31][39]. After firing LnBaCo2O5+δ double perovskites at 850 °C in air for durations ranging from 60 to 100 h, no impurity phases or phase transitions were detected. This finding highlights the remarkable structural stability of these oxides under the standard operating conditions of SOFCs [34,77][28][32]. Additionally, the electrical conductivities of LnBaCo2O5+δ compounds tend to increase with growth in the size of the Ln ion, leading to a rise in the number of electronic holes created by interstitial oxygen [75,76][30][31]. The electrical conductivity values of these materials surpass 100 S cm−1 between 100 and 800 °C in air, meeting the electrical conductivity requirements for SOFC cathodes [34,75,76,77][28][30][31][32]. What is more, oxides with larger Ln ions exhibit superior electrochemical performance, stemming from enhanced oxygen transport and exchange rates [34,75][28][30]. For instance, as the Ln ion shifts from Gd3+ to La3+, the maximum power density (PPD) values of SOFCs utilizing these double perovskite cathodes increase from 443 to 516 mW cm2 [75][30].
Despite the numerous advantages of LnBaCo2O5+δ as a cathode catalyst for SOFCs, there are certain technical challenges that require further improvements. Firstly, enhancing the catalytic activity of these oxides for ORR is paramount. Chen et al. [74][29] observed that the ASR of PrBaCo2O5+δ on SDC electrolytes increases from 0.18 to 5.68 Ω cm2 as the temperature drops from 650 to 500 °C. Moreover, the PPD of SOFCs utilizing PrBaCo2O5+δ as the cathode material decreases from 866 mW cm2 (at 650 °C) to 115 mW cm2 (at 500 °C). Secondly, it is essential to minimize the thermal mismatch between these cobalt-based cathode materials and other SOFC components. Kim et al. [75][30] reported that the thermal expansion coefficients (TECs) of LnBaCo2O5+δ double perovskites increase from 16.6 × 10−6 K−1 (Ln = Gd3+) to 24.3 × 10−6 K−1 (Ln = Pr3+) with larger Ln sizes at 80–900 °C. Given that the TECs of standard electrolytes for SOFCs, such as GDC, SDC, and LSGM, are around 11 × 10−6 K−1, this notable thermal mismatch between LnBaCo2O5+δ and the electrolyte could adversely affect fuel cell stability. Thirdly, tuning the physicochemical properties of the surface is essential. The surface physicochemical properties serving as catalysts for the ORR significantly influence cathode performance. Findings by Téllez et al. [79][34] suggest that the surface composition and morphology of LnBaCo2O5+δ (Ln = Pr, Gd) double perovskites are profoundly influenced by exposure time, temperature, and ambient atmosphere. A quick covering of the electrocatalytic transition metal by inactive Ln3+ or Ba2+ cations, observed under certain conditions, can be detrimental to the ORR. Therefore, the subsequent sections will provide a comprehensive overview of advancements in studying the physicochemical property attributes of double perovskites and in adjusting the composition and nanostructure of LnBaCo2O5+δ.
2. Nanostructure and Nanoscience of LnBaCo2O5+δ
Nanostructures offer significantly enhanced surface area-to-volume ratios and expanded interphase and interfacial areas. As such, they have the potential to augment electrochemical reaction sites. Perovskite oxides with nanostructured morphologies have been rigorously studied and employed in solid oxide fuel cells [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56] as well as other energy-related applications [199,200,201,202][57][58][59][60]. Reducing the operating temperature creates an opportunity to use nanostructured materials, which can sidestep the slow ORR and, in turn, boost the catalytic performance of the cathode [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56]. Infiltration is a common and straightforward method for developing nanostructured cathode materials tailored for SOFCs [182,183,184][40][41][42]. A nanostructured cathode material, represented by the formula SmBa0.5Sr0.5Co2O5+δ, was created by infusing its precursor solution into the porous LSGM framework, followed by calcining at 850 °C. This material showcased commendable electrochemical performance [185][43]. For instance, it showed an ASR as low as 0.12 Ω cm2 and a PPD of up to 0.70 W cm−2 at 500 °C. Electrospinning, praised for its scalability and precision, was utilized to fabricate a GdBaCo2O5+δ cathode material possessing a nanofiber configuration, achieving a comparatively low ASR, approximately 0.10 Ω cm2 at 700 °C [194][52].
Ding et al. [185][43] managed to produce unique needle-like nanospikes of the cathode material PrBaCo2O5+δ by applying a discharge voltage of 0.1 V to the anode-supported single cell, arranged as NiO-Sm0.2Ce0.8O1.9/Sm0.2Ce0.8O1.9/PrBaCo2O5+δ, and then firing the PrBaCo2O5+δ cathode slurry at 450 °C. These nanospikes, with an average diameter of 20 nm and lengths spanning from tens to hundreds of nanometers, are uniformly distributed along the pore boundaries of the porous cathode. For the single cell that used the nanospikes PrBaCo2O5+δ as the cathode, exceptionally high maximum power densities of 1.453 W cm−2 at 550 °C and 1.044 W cm−2 at 500 °C, coupled with excellent endurance, were recorded.
The fabrication of double perovskites in a thin-film architecture not only facilitates fundamental studies to evaluate inherent properties of materials [73,183,203,204][27][41][61][62] but also illuminates a new avenue for the development of high-performing cathode materials [72,186,188][26][44][46]. The influence of orientations on the electrochemical performance of double perovskites was appraised by Gao et al. [186][44]. They produced PrBaCo2O5+δ thin films with different orientations, including (110), (001), and (111), using pulsed laser deposition. The thin film with the (111) orientation showed superior performance, achieving an ASR of 0.302 Ω cm² at 600 °C. Liu et al. [187,188][45][46] fabricated symmetric half-cells by coupling single-crystal, highly epitaxial LnBaCo2O5+δ (Ln = Pr, La) thin-film cathodes with Gd0.8Ce0.2O2:Y0.08Zr0.92O2 electrolytes and subsequently characterized their oxygen surface exchange and catalytic activity. For instance, the symmetric half-cell featuring the epitaxial LaBaCo2O5+δ thin film displayed remarkable properties, such as an impressively fast surface exchange rate of 0.017 cm s−1 at 600 °C and an exceptionally low activation energy value of 0.49 eV. These outcomes might be ascribed to the structural entropy arising from the nano-ordered oxygen vacancy framework.
References
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl. Phys. Lett. 2005, 86, 091910.
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Transport and magnetic properties of GdBaCo2O5+δ single crystals: A cobalt oxide with square-lattice CoO2 planes over a wide range of electron and hole doping. Phys. Rev. B 2005, 71, 134414.
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505.
- Kim, G.; Wang, S.; Jacobson, A.J.; Yang, Z.; Donner, W.; Chen, C.L.; Reimus, L.; Brodersen, P.; Mims, C.A. Oxygen exchange kinetics of epitaxial PrBaCo2O5+δ thin films. Appl. Phys. Lett. 2006, 88, 024103.
- Tarancόn, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813.
- Tsvetkov, D.S.; Ananjev, M.V.; Eremin, V.A.; Zuev, A.Y.; Kurumchin, E.K. Oxygen nonstoichiometry, defect structure and oxygen diffusion in the double perovskite GdBaCo2O6−δ. Dalton Trans. 2014, 43, 15937–15943.
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310.
- Parfitt, D.; Chroneos, A.; Tarancόn, A.; Kilner, J.A. Oxygen ion diffusion in cation ordered/disordered GdBaCo2O5+δ. J. Mater. Chem. 2011, 21, 2183–2186.
- Tarancόn, A.; Chroneos, A.; Parfitt, D.; Kilner, J. Oxygen diffusion in ordered/disordered double perovskites. Ecs Trans. 2011, 35, 1151–1154.
- Hermet, J.; Geneste, G.; Dezanneau, G. Molecular dynamics simulations of oxygen diffusion in GdBaCo2O5.5. Appl. Phys. Lett. 2010, 97, 174102.
- Shiiba, H.; Nakayama, M.; Kasuga, T.; Grimes, R.W.; Kilner, J.A. Calculation of arrangement of oxygen ions and vacancies in double perovskite GdBaCo2O5+δ by first-principles DFT with monte carlo simulations. Phys. Chem. Chem. Phys. 2013, 15, 10494–10499.
- Zapata, J.; Burriel, M.; Carcía, P.; Kilner, J.A.; Santiso, J. Anisotropic O18 tracer diffusion in epitaxial films of GdBaCo2O5+δ cathode material with different orientations. J. Mater. Chem. A 2013, 1, 7408–7414.
- Seymour, I.D.; Tarancόn, A.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ionics 2012, 216, 41–43.
- Burriel, M.; Peña-Martínez, J.; Chater, R.J.; Fearn, S.; Berenov, A.V.; Skinner, S.J.; Kilner, J.A. Anisotropic oxygen ion diffusion in layered PrBaCo2O5+δ. Chem. Mater. 2012, 24, 613–621.
- Chen, Y.-C.; Yashima, M.; Peña-Martínez, J.; Kilner, J.A. Experimental visualization of the diffusional pathway of oxide ions in a layered perovskite-type cobaltite PrBaCo2O5+δ. Chem. Mater. 2013, 25, 2638–2641.
- Cox-Galhotra, R.A.; Huq, A.; Hodges, J.P.; Yu, C.; Wang, X.; Gong, W.; Jacobson, A.J.; McIntosh, S. An in-situ neutron diffraction study of the crystal structure of PrBaCo2O5+δ at high temperature and controlled oxygen partial pressure. Solid State Ionics 2013, 249–250, 34–40.
- Hu, Y.; Hernandez, O.; Broux, T.; Bahout, M.; Hermet, J.; Ottochian, A.; Ritter, C.; Geneste, G.; Dezanneau, G. Oxygen diffusion mechanism in the mixed ion-electron conductor NdBaCo2O5+x. J. Mater. Chem. 2012, 22, 18744–18747.
- Cox-Galhotra, R.A.; Huq, A.; Hodges, J.P.; Kim, J.-H.; Yu, C.; Wang, X.; Jacobson, A.J.; Mcintosh, S. Visualizing oxygen anion transport pathways in NdBaCo2O5+δ by in situ neutron diffraction. J. Mater. Chem. 2013, 1, 3091–3100.
- Hermet, J.; Dupé, B.; Dezanneau, G. Simulations of REBaCo2O5.5 (RE = Gd, La, Y) cathode materials through energy minimization and molecular dynamics. Solid State Ionics 2012, 216, 50–53.
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Defect structure and charge transfer in the double perovskite GdBaCo2O6−δ. Solid State Ionics 2011, 192, 215–219.
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-site cation ordering on chemical stability, oxygen stoichiometry and electrical conductivity in layered LaBaCo2O5 double perovskite. Materials 2016, 9, 154.
- Anjum, U.; Khan, T.S.; Agarwal, M.; Haider, M.A. Identifying the origin of the limiting process in a double perovskite PrBa0.5Sr0.5Co1.5Fe0.5O5+δ Thin-film electrode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 25243–25253.
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ionics 2010, 180, 1620–1625.
- Pang, S.; Wang, W.; Su, Y.; Shen, X.; Wang, Y.; Xu, K.; Chen, C. Synergistic effect of A-site cation ordered-disordered perovskite as a cathode material for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2017, 164, F775–F780.
- Zhou, Y.; Lü, Z.; Xu, S.; Wei, B.; Xu, D.; Yang, Z. The electronic structure and the oxygen adsorption at BaO terminated surface of GdBaCo2O5.5: A first principles study. Solid State Commun. 2020, 311, 113871.
- Wang, H.B.; Bao, S.Y.; Liu, J.; Collins, G.; Ma, C.R.; Liu, M.; Chen, C.L.; Dong, C.; Whangbo, M.-H.; Guo, H.M.; et al. Ultrafast chemical dynamic behavior in highly epitaxial LaBaCo2O5+δ thin films. J. Mater. Chem. C 2014, 2, 5660–5666.
- Subardi, A.; Liao, K.Y.; Fu, Y.P. Oxygen transport, thermal and electrochemical properties of NdBa0.5Sr0.5Co2O5+δ cathode for SOFCs. J. Eur. Ceram. Soc. 2019, 39, 30–40.
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889.
- Chen, D.J.; Ran, R.; Zhang, K.; Wang, J.; Shao, Z.P. Intermediate-temperature electrochemical performance of a polycrystalline PrBaCo2O5+δ cathode on samarium-doped ceria electrolyte. J. Power Sources 2009, 188, 96–105.
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+δ oxides as cathodes for intermediate-temperature solid oxide fuel cells. J. Electrochem. Soc. 2008, 155, B385–B390.
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Su, Z.X.; Xu, H.X.; Xu, Q.L.; Chen, C.L. Characterization of cation-ordered perovskite oxide LaBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 6836–6843.
- Pang, S.L.; Jiang, X.N.; Wang, Q.X.N.; Zhang, Q.Y. Structural stability and high-temperature electrical properties of cation-oredered/disordered perovskite LaBaCoO. Mater. Chem. Phys. 2012, 131, 642–646.
- Chen, D.J.; Ran, R.; Shao, Z.P. Effect of firing temperature on the microstructure and performance of PrBaCo2O5+δ cathodes on Sm0.2Ce0.8O1.9 electrolytes fabricated by spray deposition-firing processes. J. Power Sources 2010, 195, 4667–4675.
- Téllez, H.; Druce, J.; Ju, Y.-W.; Kilner, J.; Ishihara, T. Surface chemistry evolution in LnBaCo2O5+δ double perovskites for oxygen electrodes. Int. J. Hydrogen Energy 2014, 39, 20856–20863.
- Muñoz-Gil, D.; Pérez-Coll, D.; Peña-Martínez, J.; Garcia-Martín, S. New insights into the GdBaCo2O5+δ material: Crystal structure, electrical and electrochemical properties. J. Power Sources 2014, 263, 90–97.
- Ishizawa, N.; Asaka, T.; Kudo, T.; Fukuda, K.; Yasuhara, A.; Abe, N.; Arima, T. Structural evolution of GdBaCo2O5+δ (δ = 7/18) at elevated temperatures. Chem. Mater. 2014, 26, 6503–6517.
- Aksenova, T.V.; Gavrilova, L.Y.; Yaremchenko, A.A.; Cherepanov, V.A.; Kharton, V.V. Oxygen nonstoichiometry, thermal expansion and high-temperature electrical properties of layered NdBaCo2O5+δ and SmBaCo2O5+δ. Mater. Res. Bull. 2010, 45, 1288–1292.
- Shi, Z.; Xia, T.; Meng, F.; Wang, J.; Lian, J.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C.; Meng, J. A layered perovskite EuBaCo2O5+δ for intermediate-temperature solid oxide fuel cell cathode. Fuel Cells 2013, 14, 979–990.
- Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6-δ-Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells: Stability and cation interdiffusion. Energies 2019, 12, 417.
- Ding, D.; Li, X.X.; Lai, S.Y.; Gerdes, K.; Liu, M.L. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575.
- Choi, Y.; Choi, S.; Jeong, H.Y.; Liu, M.L.; Kim, B.-S.; Kim, G. Highly efficient layer-by-layer-assisted infiltration for high-performance and cost-effective fabrication of nanoelectrodes. ACS Appl. Mater. Interfaces 2014, 6, 17352–17357.
- Han, D.; Wu, H.; Li, J.L.; Wang, S.R.; Zhan, Z.L. Nanostructuring of SmBa0.5Sr0.5Co2O5+δ cathodes for reduced-temperature solid oxide fuel cells. J. Power Sources 2014, 246, 409–416.
- Ding, H.P.; Xue, X.J. An Interfacial nanospike-structured cathode for low temperature solid oxide fuel cells. Adv. Mater. Interfaces 2014, 1, 1400008.
- Gao, Y.; Chen, D.J.; Chen, C.; Shao, Z.P.; Ciucci, F. Oriented PrBaCo2O5+δ thin films for solid oxide fuel cells. J. Power Sources 2015, 278, 623–629.
- Liu, J.; Collins, G.; Liu, M.; Chen, C.L. Superfast oxygen exchange kinetics on highly epitaxial LaBaCo2O5+δ thin films for intermediate temperature solid oxide fuel cells. APL Mater. 2013, 1, 031101.
- Liu, J.; Collins, G.; Liu, M.; Chen, C.L.; He, J.; Jiang, J.C.; Meletis, E.I. Ultrafast oxygen exchange kinetics on highly epitaxial PrBaCo2O5+δ thin films. Appl. Phys. Lett. 2012, 100, 193903.
- Pang, S.; Long, C.; Tang, X.; Fang, T.; Ke, L.; Yang, G.; Song, Y.; Chen, C. Highly active and robust biomimetic ceramic catalyst for oxygen reduction reaction: Inspired by plant leaves. Ceram. Int. 2023, 49, 20273–20280.
- Kim, S.; Jun, A.; Kwon, O.; Kim, J.; Yoo, S.; Jeong, H.Y.; Shin, J.; Kim, G. Nanostructured double perovskite cathode with low sintering temperature for intermediate temperature solid oxide fuel cells. ChemSusChem 2015, 8, 3153–3158.
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Zuev, A.Y. Oxygen content, cobalt oxide exsolution and defect structure of the double perovskite PrBaCo2O6-δ. J. Mater. Chem. A 2016, 4, 1962–1969.
- Pang, S.; Song, Y.; Cui, M.; Tang, X.; Long, C.; Ke, L.; Yang, G.; Fang, T.; Guan, Y.; Chen, C. Rapid and durable oxygen reduction reaction enabled by a perovskite oxide with self-cleaning surface. J. Energy Chem. 2023, 83, 333–340.
- Fu, M.; Lin, X.; Tan, L.; Zhang, P.; Xie, H.; Tao, Z. Self-assembled Fe-doped PrBaCo2O5+δ composite cathodes with disorder transition region for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2023, 48, 15229–15237.
- Jiang, X.N.; Xu, H.X.; Wang, Q.; Jiang, L.; Li, X.N.; Xu, Q.L.; Shi, Y.C.; Zhang, Q.Y. Fabrication of GdBaCo2O5+δ cathode using electrospun composite nanofibers and its improved electrochemical performance. J. Alloys Compd. 2013, 557, 184–189.
- Hedayat, N.; Du, Y.; Ilkhani, H. Review on fabrication techniques for porous electrodes of solid oxide fuel cells by sacrificial template methods. Renew. Sust. Energ. Rev. 2017, 77, 1221–1239.
- Chen, Y.; Bu, Y.; Zhao, B.; Zhang, Y.; Ding, D.; Hu, R.; Wei, T.; Rainwater, B.; Ding, Y.; Chen, F.; et al. A durable, high-performance hollow-nanofiber cathode for intermediate-temperature fuel cells. Nano Energy 2016, 26, 90–99.
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176.
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 2017, 29, 1700132.
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071.
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637.
- Zhen, D.; Zhao, B.; Shin, H.-C.; Bu, Y.; Ding, Y.; He, G.; Liu, M. Electrospun porous perovskite La0.6Sr0.4Co1–xFexO3–δ nanofibers for efficient oxygen evolution reaction. Adv. Mater. Interfaces 2017, 4, 1700146.
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Amal, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553.
- Zou, Q.; Liu, M.; Wang, G.Q.; Lu, H.L.; Yang, T.Z.; Guo, H.M.; Ma, C.R.; Xu, X.; Zhang, M.H.; Jiang, J.C.; et al. Step terrace tuned anisotropic transport properties of highly epitaxial LaBaCo2O5.5+δ thin films on vicinal SrTiO3 substrates. ACS Appl. Mater. Interfaces 2014, 6, 6704–6708.
- Liu, J.; Liu, M.; Collins, G.; Chen, C.L.; Jiang, X.N.; Gong, W.Q.; Jacobson, A.J.; He, J.; Jiang, J.C.; Meletis, E.I. Epitaxial nature and transport properties in (LaBa)Co2O5+δ thin films. Chem. Mater. 2010, 22, 799–802.
More
