Bio-oils are produced from biomass using three main processing techniques, namely (i) organic solvent liquefaction, which involves the utilization of organic solvents at relatively low temperatures to liquefy biomass; (ii) fast pyrolysis, which involves the liquefaction of biomass at elevated temperatures in the absence of oxygen and a solvent; and (iii) hydrothermal liquefaction, which breaks down biomass in water at elevated pressures and temperatures . It is noteworthy that even though the products from the abovementioned three liquefaction processes are all called bio-oil, the properties of these bio-oils, such as hydroxyl number, chemical composition, and molecular weight, differ greatly. Therefore, the final physical properties of bio-oil-based epoxy resins depend on the processing conditions used for the corresponding bio-oils.
- bio-oil
- epoxy resin
- thermochemical processing
- sustainable material
1. Introduction
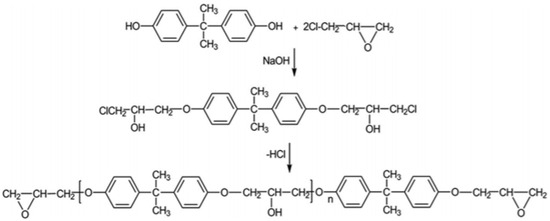
2. Bio-Based Epoxy Resins
3. Organic Solvent Liquefaction
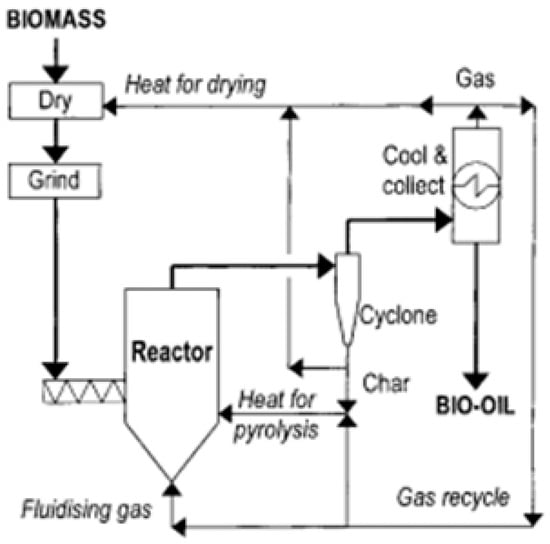
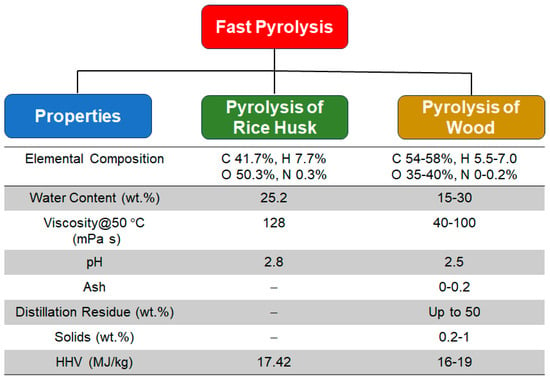
4. Fast Pyrolysis
5. Hydrothermal Liquefaction
| Feedstock | Temperature (°C) | Pressure (MPa) | Time (s) | Yield of Oil (%) | Calorific Value (MJ/Kg) |
Reference |
|---|---|---|---|---|---|---|
| Dairy Manure | 250–380 | 10–34 | – | 50 | – | [29] |
| Sewage Sludge | 300 | 10 | 30–1200 | 48 | 37–39 | [30] |
| Rubbish | 250–340 | 6–8 | 360–7200 | 27.6 | 36 | [31] |
| Sewage Sludge | 150–300 | 8–20 | 0–10,800 | 44.5 | 35.7 | [32] |
| Sewage Sludge | 250–350 | 8–20 | – | 30.7 | 36.4 | [25] |
| Municipal Solid Waste | 260–340 | 13–24 | – | 32 | 46 | [33] |
| Municipal Solid Waste | 295–450 | – | 1200–5400 | 35–63.3 | – | [34] |
| Sewage Sludge | 300–360 | 10–18 | 5–20 | – | 30–35 | [35] |
| Swine Manure | 305 | 10.3 | 80 | 70 | 25–33 | [36] |
References
- May, C.A. Epoxy Resins: Chemistry and Technology, 2nd ed.; Marcel Dekker: New York, NY, USA, 1988; p. 1.
- Bilyeu, B.; Brostow, W.; Menard, K.P. Epoxy thermosets and their applications I: Chemical structures and applications. J. Mater. Educ. 1999, 21, 281–286.
- Ueki, T.; Nishijima, S.; Izumi, Y. Designing of epoxy resin systems for cryogenic use. Cryogenics 2005, 45, 141–148.
- Karnati, S.R.; Agbo, P.; Zhang, L. Applications of silica nanoparticles in glass/carbon fiber-reinforced epoxy nanocomposite. Compos. Commun. 2020, 17, 32–41.
- Celikbag, Y.; Meadows, S.; Barde, M.; Adhikari, S.; Buschle-Diller, G.; Auad, M.L.; Via, B.K. Synthesis and characterization of bio-oil-based self-curing epoxy resin. Ind. Eng. Chem. Res. 2017, 56, 9389–9400.
- Ellis, B. Introduction to the chemistry, synthesis, manufacture and characterization of epoxy resins. In Chemistry and Technology of Epoxy Resins; Springer Science + Business Media: Dordrecht, The Netherlands, 1993; pp. 1–36.
- Liu, J.-Q.; Bai, C.; Jia, D.-D.; Liu, W.-L.; He, F.-Y.; Liu, Q.-Z.; Yao, J.-S.; Wang, X.-Q.; Wu, Y.-Z. Design and fabrication of a novel superhydrophobic surface based on a copolymer of styrene and bisphenol A diglycidyl ether monoacrylate. RSC Adv. 2014, 4, 18025–18032.
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw-Hill: New York, NY, USA, 2006; pp. 1–26.
- Gandini, A.; Belgacem, M.N.. The State of the Art. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N.; Gandini, A., Eds.; Elsevier: Amsterdam, 2008; pp. 1-16.
- Zhang, Q.; Philips, H.R.; Purchel, A.; Hexum, J.K.; Reineke, T.M. Sustainable and degradable epoxy resins from trehalose, cyclodextrin, and soybean oil yield tunable mechanical performance and cell adhesion. ACS Sustain. Chem. Eng. 2018, 6, 14967-14978.
- Ortiz, P.; Vendamme, R.; Eevers, W. Fully biobased epoxy resins from fatty acids and lignin.. Molecules 2020, 25, 1158.
- Naik, N.; Shivamurthy, B.; Thimmappa, B.H.S.; Guo, Z.; Bhat, R. Bio-based epoxies: Mechanical characterization and their applicability in the development of eco-friendly composites.. J. Compos. Sci. 2022, 6, 294.
- Pan, H. Synthesis of polymers from organic solvent liquefied biomass: A review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463.
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646.
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378.
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982.
- Lange, J.-P. Lignocellulose liquefaction to biocrude: A tutorial review. ChemSusChem 2018, 11, 997–1014.
- Liang, L.; Mao, Z.; Li, Y.; Wan, C.; Wang, T.; Zhang, L.; Zhang, L. Liquefaction of crop residues for polyol production. BioResources 2006, 1, 248–256.
- Czernik, S.; Bridgwater, A. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598.
- Bridgwater, A.; Peacocke, G. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73.
- Celikbag, Y.; Robinson, T.J.; Via, B.K.; Adhikari, S.; Auad, M.L. Pyrolysis oil substituted epoxy resin: Improved ratio optimization and crosslinking efficiency. J. Appl. Polym. Sci. 2015, 132, 42239.
- Lu, Q.; Li, W.-Z.; Zhu, X.-F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383.
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72.
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156.
- Xiu, S.; Shahbazi, A.; Shirley, V.; Cheng, D. Hydrothermal pyrolysis of swine manure to bio-oil: Effects of operating parameters on products yield and characterization of bio-oil. J. Anal. Appl. Pyrolysis 2010, 88, 73–79.
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624.
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly efficient liquefaction of woody biomass in hot-compressed alcohol- water co-solvents. Energy Fuels 2010, 24, 4659–4667.
- Celikbag, Y.; Via, B.K.; Adhikari, S.; Buschle-Diller, G.; Auad, M.L. The effect of ethanol on hydroxyl and carbonyl groups in biopolyol produced by hydrothermal liquefaction of loblolly pine: 31P-NMR and 19F-NMR analysis. Bioresour. Technol. 2016, 214, 37–44.
- Ogi, T.; Yokoyama, S.-Y.; Koguchi, K. Direct liquefaction of wood by alkali and alkaline earth salt in an aqueous phase. Chem. Lett. 1985, 14, 1199–1202.
- Itoh, S.; Suzuki, A.; Nakamura, T.; Yokoyama, S.-Y. Production of heavy oil from sewage sludge by direct thermochemical liquefaction. Desalination 1994, 98, 127–133.
- Minowa, T.; Murakami, M.; Dote, Y.; Ogi, T.; Yokoyama, S.-Y. Oil production from garbage by thermochemical liquefaction. Biomass Bioenergy 1995, 8, 117–120.
- Suzuki, A.; Nakamura, T.; Yokoyama, S.-Y. Effect of operating parameters on thermochemical liquefaction of sewage sludge. J. Chem. Eng. Jpn. 1990, 23, 6–11.
- Gharieb, H.K.; Faramawy, S.; Zaki, N. Liquefaction of cellulosic waste V. Water formation and evaluation of pyrolytic char as a by-product of pyrolysis reaction. Fuel Sci. Technol. Int. 1995, 13, 895–909.
- He, B.J.; Zhang, Y.; Yin, Y.; Funk, T.L.; Riskowski, G.L. Operating temperature and retention time effects on the thermochemical conversion process of swine manure. Trans. ASAE 2000, 43, 1821.
- Balat, M. Mechanisms of thermochemical biomass conversion processes. Part 3: Reactions of liquefaction. Energy Sources Part A 2008, 30, 649–659.
- He, B.J.; Zhang, Y.; Funk, T.L.; Riskowski, G.L.; Yin, Y. Thermochemical conversion of swine manure: An alternative process for waste treatment and renewable energy production. Trans. ASAE 2000, 43, 1827.