Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aakash Rai Gupta and Version 2 by Rita Xu.
Ischemia with no obstructive coronary arteries (INOCA) is a relatively newly discovered ischemic phenotype that affects patients similarly to obstructive coronary artery disease (CAD) but has a unique pathophysiology and epidemiology. Patients with INOCA present with ischemic signs and symptoms but no obstructive CAD seen on coronary CTA or invasive coronary angiography, which can assess epicardial vessels.
- INOCA
- CMR
- ischemia with no obstructive coronary arteries
1. Introduction
First discovered in 1973 by Harvey Kemp [1], ischemia with no obstructive coronary arteries (INOCA) refers to patients with stable ischemic symptoms and visually normal or non-obstructive coronary arteries (i.e., <50% reduction in coronary artery diameter appreciated on invasive or CT angiography) [2]. The mechanism of INOCA involves coronary microvascular dysfunction and/or coronary vasospasm, which is explained further below. Though initially considered benign, INOCA is now known to represent true cardiac disease and increases morbidity and mortality. For accurate diagnosis and risk stratification of these patients, a combination of anatomical and functional testing of the epicardial arteries and the microvasculature is crucial [3]. However, diagnostic tests remain underutilized in INOCA [3]; as the current gold standard, coronary reactivity testing is invasive, expensive, and technically challenging [4][5][4,5]. Fortunately, non-invasive perfusion imaging techniques are emerging that can provide comparably accurate vasomotor measurements and lower the barrier for evaluation [3][6][7][3,6,7].
2. Epidemiology, Prevalence, Risk Factors, and Outcomes of INOCA
INOCA is a relatively common condition, affecting 3 to 4 million individuals in the United States alone [8]. Among patients undergoing coronary angiography for suspected angina, approximately 60% of women and 30% of men have INOCA [8]. It is thought that this demographic disparity in the prevalence of INOCA is due to sex-related differences in normal cardiac physiology as well as a heterogenous representation of risk factors in women compared to men [8]. There are several risk factors associated with INOCA, including traditional cardiovascular risk factors (i.e., hypertension, diabetes, hyperlipidemia, age, and smoking), non-traditional risk factors (i.e., psychosocial stress, autoimmune disorders, and hormonal changes), female sex, and postmenopausal status [9][10][9,10]. There is also growing evidence for drug-induced INOCA, including one study that found microvascular disease associated with the use of anthracycline [11]. Several studies have highlighted the significant morbidity and mortality associated with INOCA, including an increased risk of major adverse cardiac events (MACE), heart failure with preserved ejection fraction (HFpEF), stroke, and coronary microvascular dysfunction (CMD) [9][10][9,10]. INOCA has been found to increase the risk of MACE 1.5–1.8-fold and the risk of all-cause mortality 1.3–1.5-fold [9][10][12][9,10,12]. The WISE study demonstrated that symptomatic women with INOCA experienced a 10-fold increase in heart failure hospitalizations compared to healthy asymptomatic women [10]. Overall, INOCA reduces a patient’s quality of life with increased symptom burden, cardiac anxiety, emergency room visits, and repeated testing with invasive angiography [13][14][13,14]. INOCA also comprises a substantial healthcare burden, with costs comparable to obstructive CAD [9]. It accounts for almost half of all angiography procedures [10][15][10,15]. Unfortunately, INOCA is often under-detected and undertreated in both men and women due to limitations in theour current diagnostic tools, inadequate awareness, and bias [3]. Early identification and appropriate management of INOCA are essential to reduce the risk of adverse outcomes in affected individuals.3. INOCA Endotypes: Pathophysiology and Current Diagnostic Criteria
The coronary microvasculature (especially the small arterioles) provides a significant component of the overall coronary vascular resistance and is thereby a pivotal regulator of myocardial blood flow [16]. Disturbances in the microvascular structure and/or vasodilator responses can lead to INOCA [16]. The two endotypes of INOCA include microvascular dysfunction (MVD) and vasospastic angina (VSA). They represent distinct but frequently coexistent mechanisms [17]. A meta-analysis examining the distribution of these endotypes found that approximately 41% of INOCA cases are MVD, 40% are VSA, and 23% are a combined endotype [3]. Table 1 outlines the diagnostic criteria for each endotype and compares them to non-cardiac chest pain [18].Table 1. Diagnostic criteria for INOCA endotypes and non-cardiac chest pain.
| Diagnosis | Diagnostic Criteria |
|---|---|
| Microvascular dysfunction (MVD) * |
|
| Vasospastic angina (VSA) ** |
|
| Non-cardiac chest pain |
|
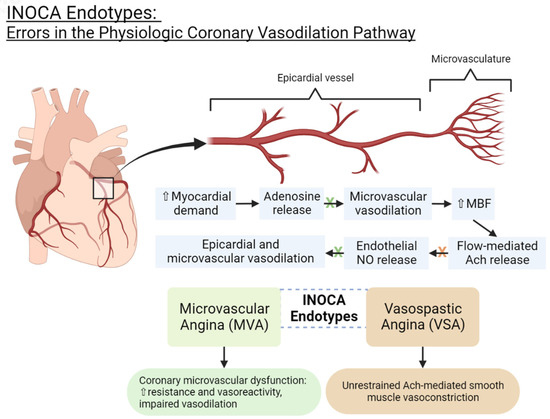
Figure 1.
INOCA endotypes: errors in the physiologic coronary vasodilation pathway. NO = nitric oxide. Ach = acetylcholine. MBF = myocardial blood flow.
