1. Acute Kidney Injury
Acute kidney injury (AKI) is a syndrome defined by a rapid increase in serum creatinine, reduced urine output (oliguria or anuria), or both. It is an essential complication in hospitalized patients, occurring in 10–15% of all hospitalizations
[1][4]. It is especially prevalent in intensive care units, where the prevalence can reach 50% or more
[2][5]. Studies have corroborated prolonged hospital stay, increased likelihood of developing CKD, and increased short- and long-term mortality in hospitalized patients with AKI
[3][6]. Furthermore, the global burden of AKI-related mortality exceeds that of breast cancer, heart failure, or diabetes mellitus, with mortality remaining high despite numerous efforts and advancements in diagnosing and managing patients with this condition
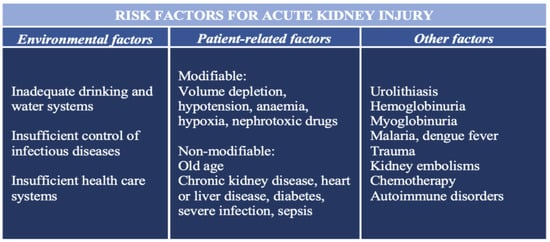
[4][7]. Several environmental, socioeconomic, and patient-related risk factors for the development of AKI are summarized in
Figure 1 [5][8].
Figure 1.
The most important risk factors for the development of acute kidney injury.
It should be remembered that AKI is a clinical term and can be due to glomerular or tubulointerstitial involvement or both. In AKI, tubular involvement is more common and is the focus of this discussion. Pathologists use descriptive pathological findings, often called acute tubular injury (ATI). Prerenal, intrarenal, and postrenal causes of AKI can all cause different degrees of ATI. Dissociation between clinical and pathological findings is not rare, especially in prerenal causes of AKI, for example, hypovolemia and hemorrhagic shock. In such cases, ATI is mild or even absent
[6][9]. At the same time, patients are often dialysis-dependent and experience several complications related to kidney failure, for example, fluid overload, electrolyte disturbances, metabolic acidosis, and uremia
[7][10].
ATI is characterized by focal or diffuse tubular luminal dilatation, simplification of the lining epithelium, loss of the brush border in proximal tubules, loss of nuclei, and the presence of nucleoli. At times, signs of epithelial cell regeneration are present (epithelial cell mitosis and cytoplasmic basophilia). In severe cases, especially in prolonged AKI, focal or diffuse tubular cell necrosis is present. In these instances, the term acute tubular necrosis (ATN) is used
[8][11].
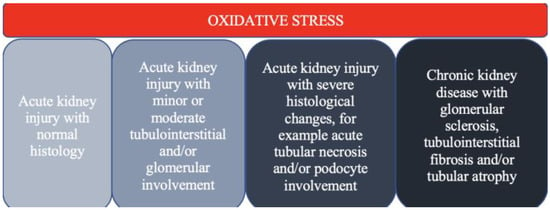
Oxidative stress is crucial in developing kidney injury, ranging from AKI to irreversible CKD
[9][12] (
Figure 2).
Figure 2.
The spectrum of kidney injury in the context of oxidative stress.
2. Oxidative Stress
2.1. The Definition and Significance of Oxidative Stress
Oxidative stress is the imbalance favoring the increased generation of ROS and/or reduced body’s innate antioxidant defense mechanisms
[10][13]. The processes of oxidation and reduction constantly happen in the cells, but the damage does not usually occur due to several protective enzymatic and non-enzymatic anti-oxidative mechanisms that keep both of these processes in balance. Whenever there is a balance shift towards increased oxidation, oxidative stress occurs
[11][14]. A pathological shift towards oxidative stress and consequent cell and tissue injury results in the modification of lipid, protein, and DNA structures, impacting their functions as well
[12][15]. In addition to causing direct tissue damage, oxidative stress can activate multiple intracellular signaling pathways and thereby indirectly induce cell apoptosis or cell overgrowth, extracellular matrix production and degradation, oxygen sensing, and inflammation, leading to significant organ dysfunction of the cardiovascular system, pancreas, kidneys, and lungs
[13][14][16,17].
Direct analysis of free radicals in an in vivo system is demanding due to their short lifespan. Additionally, measuring antioxidants is challenging, primarily because many antioxidants with different properties exist. A significant limitation is that oxidative stress is usually a focal response to tissue injury, making detecting it even more taxing. Due to this, these measurements are complex, expensive, and time-consuming
[14][17]. Studies have thus far used a plethora of different techniques of antioxidative capacity and oxidative stress, for example, total antioxidant capacity (TAC) and ferric-reducing ability of plasma (FRAP), epoxidation end-products measurements
[15][18], carbonyl derivates of amino acid residues (lysine, proline, threonine, and arginine), advanced oxidation protein products (AOPP)
[16][19], advanced glycation end products (AGEs)
[17][20], 8-oxo-2′-deoxyguanosine as a marker of oxidative DNA damage
[18][21], and 7,8-dihydro-8-deoxyguanosine as a marker of oxidative RNA damage
[19][22]. It is pivotal to point out that different features should be used to fully assess the extent of oxidative damage in the body instead of relying on a single marker
[15][18].
2.2. Oxidative Stress and Chronic Kidney Disease
Oxidative stress is commonly found in CKD and is associated with increased all-cause mortality in these patients. It is already present in the early stages of CKD and is even more apparent in patients on hemodialysis (HD) and peritoneal dialysis (PD)
[20][21][23,24]. Moreover, oxidative stress has also been observed in patients after renal transplantation
[22][25].
During the last two decades, oxidative stress has been at the center of attention as a novel, non-traditional risk factor for inflammation, atherosclerosis, diabetes mellitus (DM), and CKD progression. The heavy cardiovascular burden in patients with CKD is at least partly due to the oxidative stress observed in these patients
[23][26].
ROS in the kidneys is mainly produced by the mitochondrial respiratory chain and enzymes, such as Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase (NOX). The different NOX isoforms, including NOX1, NOX2, and NOX4, are associated with oxidative stress, worsening vascular function, and promoting interstitial fibrosis
[24][27]. Recently, NOX5 expression was found to be increased in human biopsy samples of patients with diabetic nephropathy as well
[25][28]. Furthermore, an increase in NOX5-derived ROS is essential for the faster progression of diabetic nephropathy
[26][29].
Additional oxidative markers in CKD are malondialdehyde (MDA), oxidized low-density lipoprotein (LDL), AGEs, and 8-hydroxyde-oxyguanosine
[27][30]. A decisive pathogenetic mechanism of severe cardiovascular burden in CKD patients appears to be the increased production of angiotensin II, which can cause an increase in the expression of NOX1 and NOX2 and a decrease in anti-oxidative defense mechanisms. Furthermore, a subset of antioxidant enzymes, the paraoxonases (PON), deserve special attention due to abundant clinical evidence regarding reduced serum PON1 activity in CKD as a contributor to the increased burden of cardiovascular disease
[28][31]. The increased production of ROS, especially O
2- leads to decreased production of nitric oxide (NO), an essential antioxidant protecting kidney function by increasing renal blood flow, enhancing pressure natriuresis, regulating tubuloglomerular function, and preserving fluid and electrolyte homeostasis
[29][32]. Studies have also shown a downregulation in the expression of catalase, superoxide dismutase (SOD), and NADPH dehydrogenase quinone 1 in the CKD population, all of which are important anti-oxidative enzymes
[22][25].
Diabetic kidney disease (DKD) is one of the most common causes of end-stage renal disease and is also associated with severely increased morbidity and mortality. The pathogenesis and clinical manifestations of DKD usually follow a predetermined course, beginning with albuminuria, advancing to overt proteinuria, and ultimately leading to a progressive reduction in the estimated glomerular filtration rate (eGFR). Accumulating evidence has demonstrated the overproduction of ROS as the common denominator linking the altered metabolic pathways in the kidney with disrupted renal hemodynamics known to be associated with DKD
[26][29]. Renal ROS production in DKD is predominantly mediated by various NOXs and a consequent increase in O
2-, H
2O
2, and OH. A defective antioxidant system is also important. It appears that ROS production is present not only in the podocytes and the glomerulus but also in the tubulointerstitium and is associated with pronounced and enhanced inflammation and fibrosis
[30][33].
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease. Renal manifestations of ADPKD are gradual cyst development and kidney enlargement, ultimately progressing to end-stage renal disease. Patients with ADPKD have increased serum levels of asymmetric dimethyl arginine (ADMA), MDA, and oxidized LDL cholesterol, compared to the healthy cohort. Additionally, their antioxidant capacity is reduced, which is evident from reduced plasma SOD concentrations. It appears oxidative stress plays an important role in the development and progression of kidney disease in these patients, as well
[31][34].
A major characteristic of focal segmental glomerulosclerosis (FSGS) is podocyte injury. Studies have shown podocyte injury can be at least partly attributed to oxidative stress. It appears that an increase in transforming growth factor beta (TGF-β) induces endothelin synthesis and oxidative stress in glomerular endothelium. Mitochondrial oxidative DNA damage was found even before podocyte injury
[9][32][12,35].
In patients with HD, increased concentrations of oxidative stress biomarkers have been found. Some of them are due to comorbidities and conditions accompanying these patients, such as dyslipidemia, arterial hypertension, old age, metabolic syndrome, DM, and atherosclerosis
[33][36]. Secondly, antioxidant mechanisms in these patients are impaired, as shown by reduced TAC and oxidized glutathione and reduced levels of vitamins E, C, and D
[34][37]. The use of vitamin E-coated dialyzer can decrease ROS in HD patients, reflected by the decrease in serum C-reactive protein (CRP) and interleukin-6 (IL-6)
[35][38]. Thirdly, the chronic inflammation found in HD patients is directly related to oxidative stress
[35][38].
Moreover, the HD procedure is interlinked with activating prooxidative mechanisms (for example, increased intracellular ROS, NO, markers of protein, lipid, and DNA oxidation) and the loss of antioxidative mechanisms
[36][39]. HD-related factors that cause the excessive generation of ROS are bioincompatible dialyzer membranes, heparin-based anticoagulation, prolonged use of central venous catheters instead of arteriovenous fistulas, standard HD instead of hemodiafiltration, longer duration of HD sessions, contaminated dialysate, and certain administered medications, namely, intravenous iron and erythropoietin stimulating agents
[34][37]. Studies have shown 14 times higher values of ROS after an HD session compared to the values before HD, partly due to ROS generation and partly due to antioxidative insufficiency
[37][40]. It appears that ROS generation begins shortly after starting HD (after 15 min) and then returns to its predialysis level by the end of the HD session
[38][41].
PD is more biocompatible than HD, resulting in less oxidative stress. However, PD patients still manifest oxidative stress compared to the general population and predialysis CKD patients, mainly due to the composition of the PD solution (high-glucose content, low pH, elevated osmolality, increased lactate concentration, and glucose degradation products). In addition to apparent detrimental cardiovascular effects, oxidative stress in PD patients is linked to the loss of residual renal function, peritoneal fibrosis, and ultimately, the development of encapsulating peritoneal sclerosis
[21][39][24,42].
Kidney transplantation leads to lower levels of ROS and a better preservation of anti-oxidant capacity compared to HD or PD, which is most likely one of the reasons why these patients have improved survival compared to patients with HD or PD
[40][43]. However, in kidney transplantation, factors such as immune response to allograft, ischemia/reperfusion injury, infections, and immunosuppressive therapy can be a source of significant oxidative stress
[41][44]. They can cause graft tissue damage due to the loss of nephrons and the formation of fibrosis
[42][45]. Measuring oxidative stress markers, such as MDA, is promising in predicting allograft survival and delayed graft function
[43][46]. Additional markers of oxidative stress in this population include low molecular weight advanced glycation end products (LMW-AGEs), lipid peroxidation products, advanced oxidation protein products, and plasma ADMA
[43][46].
3. The Role of Oxidative Stress in Acute Kidney Injury
Conventionally, acute kidney injury (AKI) has been viewed in an anatomical context (prerenal, renal, and postrenal), whereas, at the same time, it has been considered a risk factor for the development of subsequent CKD
[44][47]. Several pathophysiologic advances have been made in recent years that help us better understand the reciprocal interconnection between AKI and CKD. Many of these factors involve oxidative stress
[44][45][47,48].
The most important causes of AKI, especially ATN, are linked to ischemia and hypoxia. The kidneys have a sensitive system of blood flow autoregulation, which causes the GFR to be stable throughout different blood pressures. Two main mechanisms involved in maintaining renal autoregulation are tubuloglomerular feedback and myogenic response
[11][14].
In addition to nitrogen waste products, AKI is associated with elevated levels of indole and carbonyl compounds, which can upregulate systemic oxidative stress
[36][39]. NO is a vasodilator molecule formed by NO synthase (NOS), one of the most critical molecules in renal autoregulation. NO causes the vasodilatation of the afferent arteriole, leading to an increase in GFR, and it is also involved in several antifibrotic and anti-apoptotic pathways in the kidney
[46][49]. Intracellularly, low levels of NO inhibit cytochrome c-oxidase, altering the generation of ROS in the mitochondria, upregulating the hypoxia-inducible factor (HIF) in endothelial cells, and stimulating the nuclear factor erythroid 2-related factor 2 (Nrf-2). HIF and Nrf2 are renoprotective and protect the renal tissues against oxidative stress
[47][48][50,51]. Higher levels of NO interact with bound iron and can produce NO-derived reactive nitrogen species that can nitrosate thiols and lead to cell damage
[48][51].
Hall et al. studied mitochondrial structure, function, and oxidative stress in rat kidneys in response to ischemia
[49][52]. Their results showed changes in mitochondrial Nicotinamide Adenine Dinucletoide (NADH) and proton levels, followed by an upregulation in mitochondrial O
2 and disjointed mitochondria, concluding that mitochondrial dysfunction is an essential step in the early phase of ischemic renal injury
[49][52]. During ischemia, the spike in ROS drives the peroxidation of cardiolipin, a change thought to distort cristae and thereby impair efficient oxidative phosphorylation
[50][53]. In a study by Sureshbabu et al., the authors found that increased levels of ROS in AKI induce enhanced selective mitochondrial apoptosis, leading to cell injury
[51][54]. Mitochondrial dysfunction causes an increase in ROS, which, in turn, causes microvascular dysfunction as well, enhancing the effect of hypoxia on the renal medulla
[52][55]. An additional study by Tanaka et al. found changes at the lysosomal level in the renal epithelium with the loss of brush border in the proximal tubule after exposure to gentamycin, followed by an increase in markers of oxidative stress and alterations in NADH levels
[53][56].
The superoxide anion (O
2-) is generated by oxidases or inside mitochondria through the electron transport chain. Local ischemia and cytokines induced by AKI (especially in AKI associated with sepsis) activate the endothelium of renal vasculature and recruit cells from the immune system that can generate O
2- via NOX
[54][55][57,58].
Additional ROS found to be involved in AKI are hydrogen peroxide (H
2O
2) generated by dismutation or by oxidases from molecular oxygen, causing diverse cell injury and also reacting with iron-containing molecules, releasing more ROS
[11][14]; hydroxyl radical (OH) generated by the Fenton reaction, causing lipid peroxidation and an increase in other ROS
[39][42]; and hypochlorous acid (HClO), which is generated by local inflammatory cells and causes a reaction with amines, leading to the formation of toxic chloramines
[11][54][14,57]. Lipid peroxidation is also enhanced by the heme group in myoglobin in the case of rhabdomyolysis-induced AKI
[56][57][59,60].
In a study by Seija et al., the mechanisms of nitrosative stress were tested on rat kidneys in sepsis-induced AKI
[58][61]. The authors found increased protein nitration and upregulation in NOS-1 and NOS-2. They concluded that peroxynitrites are vital in developing sepsis-induced AKI
[58][61]. In Gram-negative sepsis, lipopolysaccharide (LPS) selectively binds to the Toll-like receptor (TLR), which is present in several cells, including renal tubules. The binding of LPS to TLR4 triggers a cascade reaction of events that leads to enhanced oxidative stress. Tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) promote the release of H
2O
2, with oxidative damage that further potentiates the inflammatory response
[59][62]. Several studies have shown the upregulation of TLR4 in sepsis, increasing the potential burden of selective LPS binding, consequent inflammation, and oxidative stress
[60][61][63,64]. Activating other receptors (for example, TLR9 receptors, with the binding of mitochondrial and bacterial DNA) can also lead to ROS production and oxidative stress
[62][65].
Oxidative stress is one of the most important causes of delayed graft function in patients after kidney transplant, most commonly due to ischemia–reperfusion injury. Immediately after reperfusion, a sudden increase in O
2- production inside mitochondria is noticed, leading to neutrophil activation and inflammation
[63][66]. In a study by Tanaka et al., the authors found expression of adhesion protein-1 in pericytes, a critical protein that can generate H
2O
2 and activate inflammatory cells
[53][56]. A decrease in anti-oxidant capacity has been found in mice with the loss of Nrf2 and heme oxygenase-1
[64][67]. Additionally, mice deficient in transient receptor potential melastatin-2 (TRPM-2) showed resistance to oxidative stress and apoptosis. The authors found an increase in TRPM-2 expression after renal ischemia, further indicating that oxidative stress and renal ischemia are closely linked
[65][68].
 Encyclopedia
Encyclopedia
