You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Ioannis Mavroudis and Version 2 by Lindsay Dong.
Microglial exosomes, particularly those carrying miR-124-3p, have emerged as promising candidates for therapeutic interventions in TBI. These exosomes exhibit neuroprotective effects, attenuate neuroinflammation, and promote neuronal repair and plasticity.
- traumatic brain injury
- mesenchymal stem cell-derived exosomes
- microglia
- miR-124-3p
1. Introduction
Comprehensive evidence shows that these small extracellular vesicles play a vital role in intercellular communication by transporting a wide range of bioactive molecules, including proteins, nucleic acids, and lipids [1][2][3][1,2,3]. A growing interest in them as potential therapeutic targets arose. The process through which they are generated is based on a complex cellular pathway involving the double invagination of the plasma membrane and the formation of intracellular multivesicular bodies (MVBs) that consist of intraluminal vesicles (ILVs) [4][5][6][7][8][9][4,5,6,7,8,9]. Initially, the plasma membrane invaginates to form a cup-shaped structure that includes the cell surface and soluble proteins associated with the extracellular space. Afterwards, the formation of an early-sorting endosome (ESE) is performed by direct merging with a preexisting ESE in some cases while interacting with the trans-Golgi network and endoplasmic reticulum. ESEs can mature into late-sorting endosomes, which eventually generate MVBs, also known as multivesicular endosomes. The inward invagination of the endosomal limiting membrane results in the formation of MVBs containing several ILVs that are ultimately secreted as exosomes with a size range of approximately 40 to 160 nm in diameter through MVB fusion to the plasma membrane and exocytosis [5] (Figure 1). As Kalluri et al. [4] described, the MVBs degradation can occur via two pathways: lysosomes or autophagosome-assisted degradation, or plasma membrane resorption with exosome formation (ILVs release).
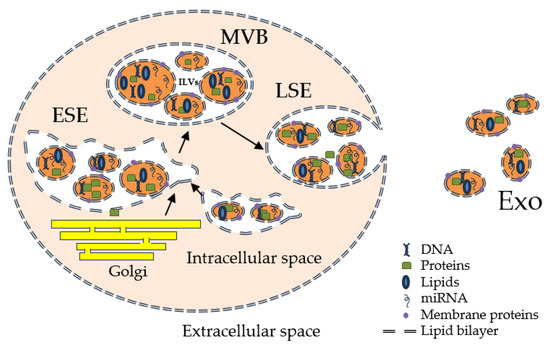
Figure 1.
Exosome biogenesis and release.
Exosomes exhibit heterogeneity that could be attributed to their size, content, functional impact on recipient cells, and cellular origin [7]. Additionally, the microenvironment and biology of cells may affect the content of exosomes and their biological markers. Exosomes can carry membrane proteins, cytosolic and nuclear proteins, extracellular matrix proteins, metabolites, and nucleic acids, such as mRNA, noncoding RNA species, and DNA [1][10][11][12][1,10,11,12]. Although exosomal cargo analyses require large pools of purified exosomes, the abundance of a given cargo in exosomes can differ, as shown by miRNA exosomal cargo [13].
Also, the functional heterogeneity that the exosomes show is associated with the recipient cells due to their variable expression of cell surface receptors, resulting in different effects, such as cell survival, apoptosis, and immunomodulation, among others, in different target cell types. The source organ or tissue of exosomes, including whether they originate from cancer cells [14], could also affect their properties, such as tropism to certain organs and uptake by specific cell types. Therefore, the complexity and heterogeneity of exosomes could be the result of a combination of these features.
Traumatic brain injury (TBI) is a major cause of death and disability worldwide, with no clinically successful treatment available to date [15][16][15,16]. TBI is commonly known as the sequela of head injuries, causing a sudden and rapid movement of the brain inside the skull [17][18][17,18]. These events could occur in sports or various types of professions, as well as in violence, accidents, or falls [19]. Penetrating or non-penetrating brain trauma could be the result of focal penetration of foreign objects, blunt force, jolts, jerks, bumps, or blasts [20]. The pathological processes underlying TBI are direct or indirect consequences of the brain trauma and include tissue deformation and focal damage (skull, meninges, or brain), as well as blood-brain barrier and brain blood flow disturbance, molecular imbalance associated with necrosis and apoptosis, oxidative stress, and inflammation [20]. In addition, the primary consequences of the brain injury further trigger a cascade of molecular events that lead to exacerbated responses to the initial damage, or neurological damage causing varied types of physical or mental disability [20][21][20,21]. The latest reports cited global estimates of 69 million TBI cases, with more than 27 million new cases annually [22][23][22,23]. There are a few reports of TBI incidence in Romania, estimating it at approximately 300 cases per 100,000 inhabitants per year [24][25][24,25], while in the UK, approximately 1.4 million patients reach the emergency departments for head injuries [26]. The treatment for TBI usually consists of rest, pain relievers, anti-seizure medication, diuretics, coma-inducing agents, or surgery, depending on the severity of the brain damage [27][28][27,28]. In addition, it implies a fastidious rehabilitation process, while some patients exhibit irrecuperable post-traumatic disabilities [22]. In this context, therapies that could prevent or limit the brain injury’s secondary consequences could be of much help in treating severe TBI patients.
2. miR-124-3p Microglial Exosomes in Traumatic Brain Injury Repair
A recent study [29][54] pointed out that microRNAs that are found within the exosomes could be potent molecular makers used in central nervous system disease diagnosis as their specific activity variation could provide additional information regarding the pathological changes in the brain tissues. Furthermore, they emphasized their role in neural regeneration and their potential to be exogenously obtained and transferred to exosomal vesicles, making them excellent therapeutic targets. Despite being a major public health concern worldwide [22][23][24][25][26][22,23,24,25,26], the lack of effective treatments increases the burden of TBI on patients and caregivers. The current treatment approaches for TBI effects are based on symptoms relief, rest, and, in more severe cases, on the modulation of brain inflammatory processes, as well as brain edema, hemorrhages, or surgery. These treatment alternatives offer a good prognosis for patients’ survival and, in combination with physical and neurological rehabilitation, show significant improvement in the quality of life and physiological functioning of the patients [27][28][27,28]. However, there are some TBI molecular impairments that could cause permanent disability for the sufferers [26]. In this context, ways to prevent the development of permanent damage or to induce tissue regeneration could be an important step in TBI management and treatment.
The potential therapeutic effects of MSC-derived and microglial exosomes have been widely studied in recent years, with promising results [30][31][32][33][34][55,56,57,58,59]. The exosomes potential to be used as therapeutic agents is given by their ability to carry proteins, lipids, mRNA, and miRNA and to be implicated in the communication between cells [35][36][60,61]. Studies have shown that MSC-derived exosomes can contribute to the therapeutic effects of MSCs in TBI and hemorrhagic shock models, promoting neurogenesis and brain remodeling [37][38][39][43,49,50]. Administration of a single dose of exosomes induces transcriptomic changes suggestive of neuroprotection, which can reduce genes associated with stroke, neuroinflammation, and non-neuronal cell proliferation, contributing to reactive gliosis. As previously emphasized by the findings regarding the TBI pathophysiological mechanisms, an important pathway that contributes to TBI’s permanent and debilitating damage is selective hippocampal damage. The hippocampal vulnerability to TBI-driven damage was previously described by Royo et al. [40][62], who showed that ipsilateral hippocampal areas are prone to manifest neuronal loss as compared to the hippocampal area contralateral to the TBI damage. It was proposed that selective hippocampal damage due to TBI is correlated with hypoxia, glutamate toxicity, hyperexcitability, and neuroinflammation [41][63]. In this context, Chen et al. [42][37] reported that intracerebroventricularly injected human adipose MSC-Exo could significantly improve hippocampal neurogenesis in a weight-drop-induced TBI rat model, thus specifically addressing this TBI effect. Recent data added even more information about the correlation between the mechanisms behind TBI by proposing possible means by which secondary injuries due to TBI are produced. Thus, besides the primary injury that could occur to the brain-blood barrier due to direct shearing following the brain injury, the secondary injury to this barrier could be the result of astrocytic dysfunction and inflammation [43][64]. Furthermore, the molecular cascade that leads to the neuroinflammatory response further enhances even more the inflammatory status within the affected brain tissues, which is also an important player in the brain-blood barrier disruption process as well as in the occurrence of oxidative stress [44][65]. Once oxidative stress is present, further impairments result regarding the processes associated with inflammation, astrocyte and microglia activation, and mitochondrial damage [44][65]. In this context, it was shown that mitochondria-associated microRNA expression could be altered in the brains of animal models of TBI, suggesting that hippocampal mitochondria respond differently to stressors as compared to cortical mitochondria [45][66].
On the other hand, some studies suggested that selective hippocampal damage due to TBI could be mediated by gap junctions and hemichannels between the glial cells. In this way, both Chen et al. [46][67] and Mayorquin et al. [47][68] described a mechanistic association between connexins and pannexins and exosome release in the hippocampal area. In a rat model of TBI, a specific connexin phosphorylation, connexin 43, leads to tissular damage propagation and glial exosome release in the ipsilateral hippocampus. In the context of miR-124-3p treatment in TBI, it was noted that in some brain tumoral cells, this microRNA gains antiproliferative abilities and travels through the connexin 43 channels [48][49][69,70]. Similarly, pannexin-1 channel inhibition was correlated with decreased neuroinflammation following TBI and hypoxic ischemia [50][71] and could be an important target in TBI treatments. In this context, MSC-derived exosomes effectively improve functional recovery in a rat model of intracerebral hemorrhage by promoting endogenous angiogenesis and neurogenesis. Another study demonstrated that incorporation of MSC-derived exosomes into hyaluronan-collagen hydrogel can achieve both mimicking of the brain matrix and steady release of exosomes, resulting in TBI repair [51][52][53][44,45,47]. Similarly, microglial exosomes have been found to have a potential therapeutic role in TBI [54][55][51,52]. They can help modulate inflammation, reduce oxidative stress, and promote neurogenesis.
Microglia are resident immune cells in the central nervous system that play an important role in the pathophysiology of TBI. Following a brain injury event, the microglia are the first immunological actors in the central nervous system that respond to injury by changing their morphology to an ameboid shape, exhibiting phagocytic activity, and releasing inflammatory agents in the surroundings [56][72]. The dual activity of microglia in inflammation was demonstrated by the description of the M1 and M2 phenotypes that exhibit opposite inflammatory activities, anti-inflammatory and pro-inflammatory, respectively [57][73]. Thus, M2 microglia can release exosomes containing cytokines, chemokines, and microRNAs, which can interact with other central nervous system cells to promote neuroprotection and repair. Of the microRNAs implicated in this process, the miR-124 family of microRNAs has been shown to promote the polarization of microglia into the M2 phenotype and hippocampal neurogenesis [58][74], as discussed before. The molecular mechanism by which miR-124 modulated M2 polarization of the microglia was by inhibiting TLR4 signaling pathway components expression, which is also implicated in hippocampal neuron loss [59][75]. This could be an important target for exosome-based therapeutic approaches in TBI treatment.
Regarding the potential of miR-124-3p to modulate cellular and functional recovery following TBI, there are several studies that demonstrate that microglial exosomes that contain increased levels of miR-124-3p are implicated in the reparatory processes within the TBI-affected brains [55][52]. Moreover, miR-124-3p was seen to be implicated in β-amyloid metabolism within the brain, thus being able to prevent the Alzheimer’s disease-like neurodegenerative-related processes seen in the long-term effects of TBI [54][51]. However, its main contribution remains neuronal inflammation and neurite outgrowth when transported into the neurons. In addition, several studies described their activity within the brain microvascular system by promoting mTOR signaling and subsequent autophagy, as well as improving the cerebral microvascular endothelial barrier [60][35].
The mechanisms of action of miR-124-3p include the potential to bind to untranslated regions of mRNAs that code for important pathway modulators, such as the mTOR pathway [55][52], the neurotrophin signaling pathway, and the cAMP signaling pathway [54][61][51,76]. Voukila [62][77] also described the binding site targets for miR-124-3p as post-transcriptional switches involved in non-neuronal splicing, activated by miR-12-3p downregulation, which could be caused by target RNA-directed miRNA degradation.
Overall, the studies suggested that exosomes derived from MSCs or other stem cells have significant advantages and could be considered a potential therapeutic approach for TBI and SCI. The main advantages consist in their effective transport and their multiple properties in neuroprotection, endogenous angiogenesis, and neurogenesis, as well as in the inhibition of apoptosis, pyroptosis, and ferroptosis.
