Malignant mesotheliomas (MM) are hard to treat malignancies with poor prognosis and high mortality rates. This cancer is highly misdiagnosed in Sub-Saharan African countries. According to literature, the incidence of MM is likely to increase particularly in low-middle-income countries (LMICs). The burden of asbestos-induced diseases was estimated to be about 231,000 per annum. Lack of awareness and implementation of regulatory frameworks to control exposure to asbestos fibers contributes to the expected increase. Exposure to asbestos fibers can lead to cancer initiation by several mechanisms. Asbestos-induced epigenetic modifications of gene expression machinery and non-coding RNAs promote cancer initiation and progression. Furthermore, microbiome–epigenetic interactions control the innate and adaptive immunity causing exacerbation of cancer progression and therapeutic resistance.
- mesothelioma
- epigenetics
- MicroRNA
- microbiome
- immune modulation
- alternative splicing
- asbestos
- therapeutic targets
1. Introduction
2. The Burden of Asbestos-Related Diseases, a Persisting Challenge
The global incidence of mesothelioma increased during the period of 1990 to 2017. Over 50% of the cases recorded were from high socio-demographic index (SDI) regions. The age-standardized incidence rate (ASIR) decreased from 1.11 to 0.17% in Southern SSA after 2000 but increased from 2.03 to 2.30% in Australasia. In this study, Zhai et al. reported that the global trends of mesothelioma vary amongst countries, but according to the authors, the incidence of mesothelioma has decreased since 1990 [17]. Screening and early detection of mesotheliomas remains a challenge; hence, the incidence of the disease is not well recorded. The lack of resources in the LMICs could be a limiting factor in terms of diagnosing and recording mesothelioma incidences and mortality rates as expected. Recently, Chimed-Ochir et al. evaluated the correlation of country-level mesothelioma burden and asbestos use with national income status. The study looked at 80 high-income countries of which 54 (68%) reported mesothelioma deaths to WHO. The low-middle-income countries (LMICs) were 78, and only 11 (14%) of these countries reported mesothelioma deaths. The other 86% of these deaths were not reported. The highest number of mesothelioma deaths were recorded by high-income and upper-middle-income countries at 29,854 (78%), whilst LMICs reported only 8534 (22%) deaths [18]. This study echoes the need for social and scientific community awareness campaigns so that the communities are aware of the dangers of occupational and household asbestos products. The companies that still produce products that contain asbestos need to be evaluated by health professionals and inspected regularly. The need to facilitate the implementation of the regulations to control asbestos exposure should be considered. Finally, simpler reliable screening methods that can be utilized even in rural areas for early detection of asbestos-related diseases should be ventured into. In LMICs such as India, the continual use of asbestos products is remarkable. Jadhav and Gawde, 2019 predict that the country will experience a high incidence of asbestos-related diseases accounting for at least 1.25 million patients diagnosed with cancer worldwide [19]. The latency of mesothelioma is long and highly variable with a range of 13–70 years meaning that although the disease is considered rare, there is a chance that the numbers are yet to increase among people who have been exposed to asbestos before the ban or implementation of regulatory measures. The socioeconomic status in rural SSA regions makes the decision to eliminate the use of asbestos difficult as transitioning from asbestos roofing to metal sheets, for example, can be costly. Owing to its long, covert pathogenesis and latency, scientists in the SSA regions do not pay much attention to the disease as it is not necessarily obvious like other cancers commonly diagnosed in the region. There is thus the need to find better ways of diagnosing the disease with consideration of the asbestos exposure situation in LMICs like SSA and India in mind. There is a possibility that more and more cases of mesothelioma will emerge. There will have to be measures implemented to ensure that these cases are not missed and efforts to improve the quality of life are undertaken. Wagner et al. reported cases of mesothelioma induced by exposure to asbestos originating from the mines. However, one of the cases was peculiar as the patient had never worked at the mines. The relatives of the patient also mentioned no previous knowledge of asbestos exposure neither through the mining industry nor factories manufacturing asbestos production [20]. Although it is a major problem, repurposing of asbestos products for household purposes in these communities is still a normal practice. For example, asbestos sheets can be used to dry animal skin or meat prepared for traditional ceremonies. The manufacturing of asbestos products used to be a lucrative business for South Africa. The discovery of its detrimental effects on human health led to the ban of the production of asbestos products in the country. It is important to note that there is still a substantial number of houses with asbestos cement roof sheets. Guidelines for the demolition of these structures have been set by the National Institute for Occupational Health (NIOH) of South Africa. The NIOH also analyzed the samples removed from the structures and measures the asbestos fibers post demolition. Although not all of the structures were demolished, 2990 samples were collected from 3/9 provinces in the country (Gauteng, Mpumalanga, and the Western Cape). A total of 1581 bulk samples were collected. The study found that 54.8% of the bulk samples contained asbestos, and 16.1% (227) of asbestos was found in air filters post demolition [21]. This is a potential indication of how asbestos fibers escape into the air during demolition; hence, the area of demolition must be well contained. Efforts to prevent asbestos-related diseases require implementation of a set of sequential measures. Ideally, this would include managing and removing the existing asbestos housing structures and asbestos-related products replacing them with alternative materials such as iron sheet roofing; exposure to occupational asbestos fibers can be limited by taking preventative measures such as training employees on asbestos exposure and related risks; in cases where exposure is unavoidable, training on how to safely handle asbestos and related products should be implemented; the use of personal protective equipment (PPE) where necessary should be encouraged; regular measurements of asbestos fibers in the air should be done. Perkins et al. reiterated the significance of enough wetting during the demolition processes as it assists with the reduction of airborne materials including asbestos fibers [22].3. The Role of miRNA in Mesothelioma
The miRNAs involved in oncogenesis pathways are reduced in MM encouraging the development of miRNAs mimics for possible reversal of cancer initiation processes [23]. Studies done on experimental models have demonstrated the possibility of reversing oncogenesis by reintroducing specific miRNAs identified as tumorigenic. For instance, treatment of MPM tumor xenografts with ectopically re-expressed miR-206 showed a significant reduction of tumor growth achieved via G1/S cell cycle arrest. The effectiveness of miR-206 was shown to be controlled by the RTK-Ras-MAPK-PI3K/Akt-CDK pathway. This effect allowed the authors to ascertain the use of CDK6 as a novel target for miR-206 [24]. Similarly, treatment with miRNA-215-5p diminished cancer growth by activation of mouse double minute 2 (MDM2)-p53 signaling pathway and resultant caspase-dependent apoptosis [25]. The miR-126 located within intron 7 of its host gene EGFL7 is downregulated in MPM. The EGFL7 S2 region methylation status was associated with significantly worse MPM patient survival. These results correlated well with the downregulation of EGFL7 transcript variant 1 and miR-126 [26]. Significant downregulation of miR-126 is strongly correlated with serum levels of vascular endothelial growth factor (VEGF) [27] and soluble mesothelin-related peptide (SMRP). SMRP was previously identified as a possible diagnostic marker for MPM [28]. Follow-up studies identified pleural effusion SMRP as a better indicator of MPM over serum SMRP [29]. The MDM2 along with other factors such as TRAIL [30]/HIF-1α [31] involved in MPM have been investigated as therapeutic targets or potential biomarkers, respectively. Identification of miRNA as potential therapeutic targets of MM has been a topic of interest for a while, these include miR-16 [32], miR-193a-3p [33], miR-17-5p in relation to KCa1.1 [34], and miR-411, which controls the expression of IL-18 [35] (Table 1). Targeting miRNAs and related pathways continue to be the topic of interest today.| miRNA/s | Origin | Status in MM | References |
|---|---|---|---|
| miR-16-5p | MM cell lines exosomes | Upregulated | [36] |
| miR-320a | Human tissue | Downregulated | [37] |
| miR-548a-3p and miR-20a | Human serum | Upregulated | [38] |
| miR-323a-3p, miR-20b-5p and miR-101-3p | Human tissue | Downregulated | [38] |
| miR-137 | Human tissue | Variable | [39] |
| MPM cell lines | Variable | ||
| miR-486 | MPM cell lines | Downregulated | [23] |
| MiR-126 | MM cell lines exosomes | Downregulated | [40] |
| miRNA-34a/b/c | Human tissue | Downregulated | [41,42,43,[4344][41][42]][44] |
| microRNA-23b | MM cell lines | Upregulated | [45] |
| miR-625-3p | Human serum extracellular vesicles | Downregulated | [46] |
| miR-206 | Human tissue | Downregulated | [24] |
| Xenografts | Downregulated | ||
| miR-18a-3p | MM cell lines | Upregulated | [47] |
4. Epigenetic miRNA as Potential Diagnostic Biomarkers and Targeted Therapies
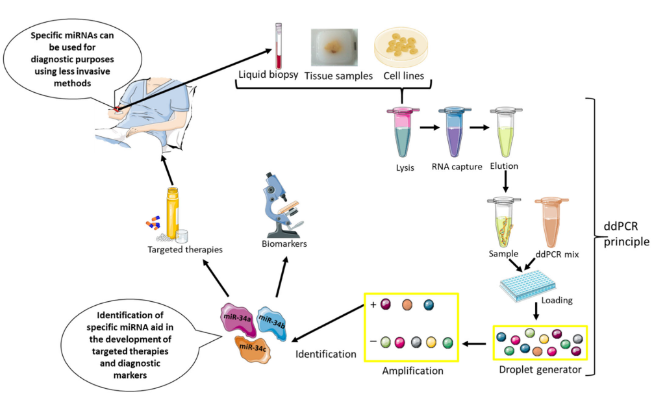
5. Epigenetic miRNA in Other Asbestos-Related Diseases, Possible Relation to MM
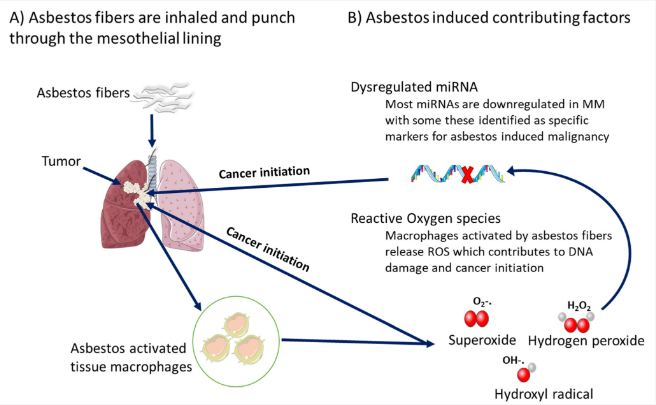
References
- Vimercati, L.; Cavone, D.; Delfino, M.C.; De Maria, L.; Caputi, A.; Ferri, G.M.; Serio, G. Asbestos exposure and malignant mesothelioma of the tunica vaginalis testis: A systematic review and the experience of the Apulia (southern Italy) mesothelioma register. Environ. Health 2019, 18, 78.
- Jain, S.; Wallen, J. Malignant Mesothelioma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519530/ (accessed on 7 April 2022).
- Larose, F.; Quigley, N.; Lacasse, Y.; Martel, S.; Lang-Lazdunski, L. Malignant pleural mesothelioma: Comparison of surgery-based trimodality therapy to medical therapy at two tertiary academic institutions. Lung Cancer 2021, 156, 151–156.
- Lam, N.S.; Van Tho, N.; Thanh, T.D.; Nakano, Y. Infectious Agents Associated with Mesothelioma. In Microbiome and Cancer; Robertson, E.S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 167–183.
- Nelson, G.; Murray, J.; Phillips, J.I. The risk of asbestos exposure in South African diamond mine workers. Ann. Occup. Hyg. 2011, 55, 569–577.
- Christensen, B.C.; Houseman, E.A.; Godleski, J.J.; Marsit, C.J.; Longacker, J.L.; Roelofs, C.R.; Karagas, M.R.; Wrensch, M.R.; Yeh, R.-F.; Nelson, H.H.; et al. Epigenetic profiles distinguish pleural mesothelioma from normal pleura and predict lung asbestos burden and clinical outcome. Cancer Res. 2009, 69, 227–234.
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56.
- Locke, W.J.; Guanzon, D.; Ma, C.; Liew, Y.J.; Duesing, K.R.; Fung, K.Y.C.; Ross, J.P. DNA Methylation Cancer Biomarkers: Translation to the Clinic. Front. Genet. 2019, 10, 1150.
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38.
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027.
- Horio, D.; Minami, T.; Kitai, H.; Ishigaki, H.; Higashiguchi, Y.; Kondo, N.; Hirota, S.; Kitajima, K.; Nakajima, Y.; Koda, Y.; et al. Tumor-associated macrophage-derived inflammatory cytokine enhances malignant potential of malignant pleural mesothelioma. Cancer Sci. 2020, 111, 2895–2906.
- Balatti, V.; Maniero, S.; Ferracin, M.; Veronese, A.; Negrini, M.; Ferrocci, G.; Martini, F.; Tognon, M.G. MicroRNAs Dysregulation in Human Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2011, 6, 844–851.
- Chivukula, R.R.; Mendell, J.T. Circular reasoning: microRNAs and cell-cycle control. Trends Biochem. Sci. 2008, 33, 474–481.
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506.
- Higuchi, R.; Goto, T.; Hirotsu, Y.; Otake, S.; Oyama, T.; Amemiya, K.; Mochizuki, H.; Omata, M. Streptococcus australis and Ralstonia pickettii as Major Microbiota in Mesotheliomas. J. Pers Med. 2021, 11, 297.
- Zhai, Z.; Ruan, J.; Zheng, Y.; Xiang, D.; Li, N.; Hu, J.; Shen, J.; Deng, Y.; Yao, J.; Zhao, P.; et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2017. JAMA Netw. Open 2021, 4, e2120360.
- Chimed-Ochir, O.; Arachi, D.; Driscoll, T.; Lin, R.-T.; Takala, J.; Takahashi, K. Burden of Mesothelioma Deaths by National Income Category: Current Status and Future Implications. Int. J. Environ. Res. Public Health 2020, 17, 6900.
- Jadhav, A.V.; Gawde, N.C. Current Asbestos Exposure and Future Need for Palliative Care in India. Indian J. Palliat. Care 2019, 25, 587–591.
- Wagner, J.C.; Sleggs, C.A.; Marchand, P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br. J. Ind. Med. 1960, 17, 260–271.
- Vorster, T.; Kgokong, N.; Phillips, J.I. Exploring the South African legacy of asbestos using routinely collected data. Occup. Health S. Afr. 2018, 24, 135–139.
- Perkins, R.A.; Hargesheimer, J.; Fourie, W. Asbestos release from whole-building demolition of buildings with asbestos-containing material. J. Occup. Env. Hyg. 2007, 4, 889–894.
- Pinelli, S.; Alinovi, R.; Poli, D.; Corradi, M.; Pelosi, G.; Tiseo, M.; Goldoni, M.; Cavallo, D.; Mozzoni, P. Overexpression of microRNA-486 affects the proliferation and chemosensitivity of mesothelioma cell lines by targeting PIM1. Int. J. Mol. Med. 2021, 47, 117.
- Singh, A.; Pruett, N.; Pahwa, R.; Mahajan, A.P.; Schrump, D.S.; Hoang, C.D. MicroRNA-206 suppresses mesothelioma progression via the Ras signaling axis. Mol. Ther. Nucleic Acids 2021, 24, 669–681.
- Singh, A.; Bhattacharyya, N.; Srivastava, A.; Pruett, N.; Ripley, R.T.; Schrump, D.S.; Hoang, C.D. MicroRNA-215-5p Treatment Suppresses Mesothelioma Progression via the MDM2-p53-Signaling Axis. Mol. Ther. J. Am. Soc. Gene. Ther. 2019, 27, 1665–1680.
- Andersen, M.; Trapani, D.; Ravn, J.; Sørensen, J.B.; Andersen, C.B.; Grauslund, M.; Santoni-Rugiu, E. Methylation-associated Silencing of microRNA-126 and its Host Gene EGFL7 in Malignant Pleural Mesothelioma. Anticancer Res. 2015, 35, 6223–6229.
- Santarelli, L.; Strafella, E.; Staffolani, S.; Amati, M.; Emanuelli, M.; Sartini, D.; Pozzi, V.; Carbonari, D.; Bracci, M.; Pignotti, E.; et al. Association of MiR-126 with soluble mesothelin-related peptides, a marker for malignant mesothelioma. PLoS ONE 2011, 6, e18232.
- Scherpereel, A.; Grigoriu, B.; Conti, M.; Gey, T.; Grégoire, M.; Copin, M.C.; Devos, P.; Chahine, B.; Porte, H.; Lassalle, P. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am. J. Respir. Crit. Care Med. 2006, 173, 1155–1160.
- Gao, R.; Wang, F.; Wang, Z.; Wu, Y.; Xu, L.; Qin, Y.; Shi, H.; Tong, Z. Diagnostic value of soluble mesothelin-related peptides in pleural effusion for malignant pleural mesothelioma: An updated meta-analysis. Medicine 2019, 98, e14979.
- Urso, L.; Cavallari, I.; Silic-Benussi, M.; Biasini, L.; Zago, G.; Calabrese, F.; Conte, P.F.; Ciminale, V.; Pasello, G. Synergistic targeting of malignant pleural mesothelioma cells by MDM2 inhibitors and TRAIL agonists. Oncotarget 2017, 8, 44232–44241.
- Pasello, G.; Urso, L.; Mencoboni, M.; Grosso, F.; Ceresoli, G.L.; Lunardi, F.; Vuljan, S.E.; Bertorelle, R.; Sacchetto, V.; Ciminale, V.; et al. MDM2 and HIF1alpha expression levels in different histologic subtypes of malignant pleural mesothelioma: Correlation with pathological and clinical data. Oncotarget 2015, 6, 42053–42066.
- Reid, G.; Pel, M.E.; Kirschner, M.B.; Cheng, Y.Y.; Mugridge, N.; Weiss, J.; Williams, M.; Wright, C.; Edelman, J.J.; Vallely, M.P.; et al. Restoring expression of miR-16: A novel approach to therapy for malignant pleural mesothelioma. Ann. Oncol. 2013, 24, 3128–3135.
- Williams, M.; Kirschner, M.B.; Cheng, Y.Y.; Hanh, J.; Weiss, J.; Mugridge, N.; Wright, C.M.; Linton, A.; Kao, S.C.; Edelman, J.J.; et al. miR-193a-3p is a potential tumor suppressor in malignant pleural mesothelioma. Oncotarget 2015, 6, 23480–23495.
- Cheng, Y.Y.; Wright, C.M.; Kirschner, M.B.; Williams, M.; Sarun, K.H.; Sytnyk, V.; Leshchynska, I.; Edelman, J.J.; Vallely, M.P.; McCaughan, B.C.; et al. KCa1.1, a calcium-activated potassium channel subunit alpha 1, is targeted by miR-17-5p and modulates cell migration in malignant pleural mesothelioma. Mol. Cancer 2016, 15, 44.
- Yamamoto, K.; Seike, M.; Takeuchi, S.; Soeno, C.; Miyanaga, A.; Noro, R.; Minegishi, Y.; Kubota, K.; Gemma, A. MiR-379/411 cluster regulates IL-18 and contributes to drug resistance in malignant pleural mesothelioma. Oncol. Rep. 2014, 32, 2365–2372.
- Munson, P.B.; Hall, E.M.; Farina, N.H.; Pass, H.I.; Shukla, A. Exosomal miR-16-5p as a target for malignant mesothelioma. Sci. Rep. 2019, 9, 11688.
- Costa, C.; Indovina, P.; Mattioli, E.; Forte, I.M.; Iannuzzi, C.A.; Luzzi, L.; Bellan, C.; De Summa, S.; Bucci, E.; Di Marzo, D.; et al. P53-regulated miR-320a targets PDL1 and is downregulated in malignant mesothelioma. Cell Death Dis. 2020, 11, 748.
- Matboli, M.; Shafei, A.E.; Azazy, A.E.; Reda, M.; El-Khazragy, N.; Nagy, A.A.; Ali, M.A.; Sobhi, M.; Abdel-Rahman, O. Clinical evaluation of circulating miR-548a-3p and -20a expression in malignant pleural mesothelioma patients. Biomark. Med. 2018, 12, 129–139.
- Johnson, T.G.; Schelch, K.; Cheng, Y.Y.; Williams, M.; Sarun, K.H.; Kirschner, M.B.; Kao, S.; Linton, A.; Klebe, S.; McCaughan, B.C.; et al. Dysregulated Expression of the MicroRNA miR-137 and Its Target YBX1 Contribute to the Invasive Characteristics of Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 258–272.
- Monaco, F.; Gaetani, S.; Alessandrini, F.; Tagliabracci, A.; Bracci, M.; Valentino, M.; Neuzil, J.; Amati, M.; Bovenzi, M.; Tomasetti, M.; et al. Exosomal transfer of miR-126 promotes the anti-tumour response in malignant mesothelioma: Role of miR-126 in cancer-stroma communication. Cancer Lett. 2019, 463, 27–36.
- Kubo, T.; Toyooka, S.; Tsukuda, K.; Sakaguchi, M.; Fukazawa, T.; Soh, J.; Asano, H.; Ueno, T.; Muraoka, T.; Yamamoto, H.; et al. Epigenetic silencing of microRNA-34b/c plays an important role in the pathogenesis of malignant pleural mesothelioma. Clin. Cancer Res. 2011, 17, 4965–4974.
- Muraoka, T.; Soh, J.; Toyooka, S.; Aoe, K.; Fujimoto, N.; Hashida, S.; Maki, Y.; Tanaka, N.; Shien, K.; Furukawa, M.; et al. The degree of microRNA-34b/c methylation in serum-circulating DNA is associated with malignant pleural mesothelioma. Lung Cancer 2013, 82, 485–490.
- Ueno, T.; Toyooka, S.; Fukazawa, T.; Kubo, T.; Soh, J.; Asano, H.; Muraoka, T.; Tanaka, N.; Maki, Y.; Shien, K.; et al. Preclinical evaluation of microRNA-34b/c delivery for malignant pleural mesothelioma. Acta Med. Okayama 2014, 68, 23–26.
- Sato, H.; Soh, J.; Aoe, K.; Fujimoto, N.; Tanaka, S.; Namba, K.; Torigoe, H.; Shien, K.; Yamamoto, H.; Tomida, S.; et al. Droplet digital PCR as a novel system for the detection of microRNA-34b/c methylation in circulating DNA in malignant pleural mesothelioma. Int. J. Oncol. 2019, 54, 2139–2148.
- Fujii, T.; Itami, H.; Uchiyama, T.; Morita, K.; Nakai, T.; Hatakeyama, K.; Sugimoto, A.; Shimada, K.; Tsuji, S.; Ohbayashi, C. HEG1-responsive microRNA-23b regulates cell proliferation in malignant mesothelioma cells. Biochem. Biophys. Res. Commun. 2020, 526, 927–933.
- Goričar, K.; Holcar, M.; Mavec, N.; Kovač, V.; Lenassi, M.; Dolžan, V. Extracellular Vesicle Enriched miR-625-3p Is Associated with Survival of Malignant Mesothelioma Patients. J. Pers. Med. 2021, 11, 1014.
- Suzuki, R.; Amatya, V.J.; Kushitani, K.; Kai, Y.; Kambara, T.; Fujii, Y.; Takeshima, Y. Inhibition of miR-18a-3p reduces proliferation of mesothelioma cells and sensitizes them to cisplatin. Oncol. Lett. 2020, 19, 4161–4168.
- He, X.X.; Kuang, S.Z.; Liao, J.Z.; Xu, C.R.; Chang, Y.; Wu, Y.L.; Gong, J.; Tian, D.A.; Guo, A.Y.; Lin, J.S. The regulation of microRNA expression by DNA methylation in hepatocellular carcinoma. Mol. Biosyst. 2015, 11, 532–539.
- Namløs, H.M.; Skårn, M.; Ahmed, D.; Grad, I.; Andresen, K.; Kresse, S.H.; Munthe, E.; Serra, M.; Scotlandi, K.; Llombart-Bosch, A.; et al. miR-486-5p expression is regulated by DNA methylation in osteosarcoma. BMC Genom. 2022, 23, 142.
- Micolucci, L.; Akhtar, M.M.; Olivieri, F.; Rippo, M.R.; Procopio, A.D. Diagnostic value of microRNAs in asbestos exposure and malignant mesothelioma: Systematic review and qualitative meta-analysis. Oncotarget 2016, 7, 58606–58637.
- Cheng, Y.Y.; Rath, E.M.; Linton, A.; Yuen, M.L.; Takahashi, K.; Lee, K. The Current Understanding Of Asbestos-Induced Epigenetic Changes Associated With Lung Cancer. Lung Cancer 2020, 11, 1–11.
- Tomasetti, M.; Gaetani, S.; Monaco, F.; Neuzil, J.; Santarelli, L. Epigenetic Regulation of miRNA Expression in Malignant Mesothelioma: miRNAs as Biomarkers of Early Diagnosis and Therapy. Front. Oncol. 2019, 9, 1293.
- Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322.
- Frontini, F.; Bononi, I.; Torreggiani, E.; Di Mauro, G.; Mazzoni, E.; Stendardo, M.; Boschetto, P.; Libener, R.; Guaschino, R.; Grosso, F.; et al. Circulating microRNA-197-3p as a potential biomarker for asbestos exposure. Sci. Rep. 2021, 11, 23955.
- Tomasetti, M.; Amati, M.; Neuzil, J.; Santarelli, L. Circulating epigenetic biomarkers in lung malignancies: From early diagnosis to therapy. Lung Cancer 2017, 107, 65–72.
- Santarelli, L.; Gaetani, S.; Monaco, F.; Bracci, M.; Valentino, M.; Amati, M.; Rubini, C.; Sabbatini, A.; Pasquini, E.; Zanotta, N.; et al. Four-miRNA Signature to Identify Asbestos-Related Lung Malignancies. Cancer Epidemiol. Biomark. Prev. 2019, 28, 119–126.
- Amatya, V.J.; Mawas, A.S.; Kushitani, K.; Mohi El-Din, M.M.; Takeshima, Y. Differential microRNA expression profiling of mesothelioma and expression analysis of miR-1 and miR-214 in mesothelioma. Int. J. Oncol. 2016, 48, 1599–1607.
- Datta, J.; Kutay, H.; Nasser, M.W.; Nuovo, G.J.; Wang, B.; Majumder, S.; Liu, C.-G.; Volinia, S.; Croce, C.M.; Schmittgen, T.D.; et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008, 68, 5049–5058.
- Xu, H.; Wu, S.; Shen, X.; Shi, Z.; Wu, D.; Yuan, Y.; Jiang, W.; Wang, Q.; Ke, Q.; Mao, Q.; et al. Methylation-mediated miR-214 regulates proliferation and drug sensitivity of renal cell carcinoma cells through targeting LIVIN. J. Cell Mol. Med. 2020, 24, 6410–6425.
- Hsieh, T.-H.; Liu, Y.-R.; Chang, T.-Y.; Liang, M.-L.; Chen, H.-H.; Wang, H.-W.; Yen, Y.; Wong, T.-T. Global DNA methylation analysis reveals miR-214-3p contributes to cisplatin resistance in pediatric intracranial nongerminomatous malignant germ cell tumors. Neuro-Oncology 2017, 20, 519–530.
