Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Kuo-Hu Chen.
Estrogen receptors (ERs) are highly expressed in osteoblasts, osteoclasts, and osteocytes, offering protective effects in bone. Osteoporosis is a serious health issue among aging postmenopausal women. The majority of postmenopausal women with osteoporosis have bone loss related to estrogen deficiency. The rapid bone loss results from an increase in bone turnover with an imbalance between bone resorption and bone formation. Osteoporosis can also result from excessive glucocorticoid usage, which induces bone demineralization with significant changes of spatial heterogeneities of bone at microscale, indicating potential risk of fracture.
- osteoporosis
- estrogen
- patients
1. Molecular Mechanisms of Actions
It is known that bone remodeling is accomplished by osteoblasts, osteoclasts, and osteocytes. The negative imbalance of bone remodeling, in which bone resorption exceeds bone formation, results in osteoporosis. At a cellular level, several mechanisms contribute to the bone loss related to estrogen deficiency (Figure 1).
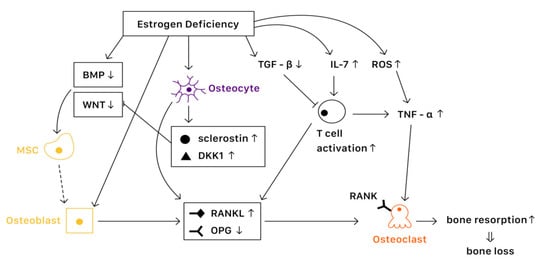
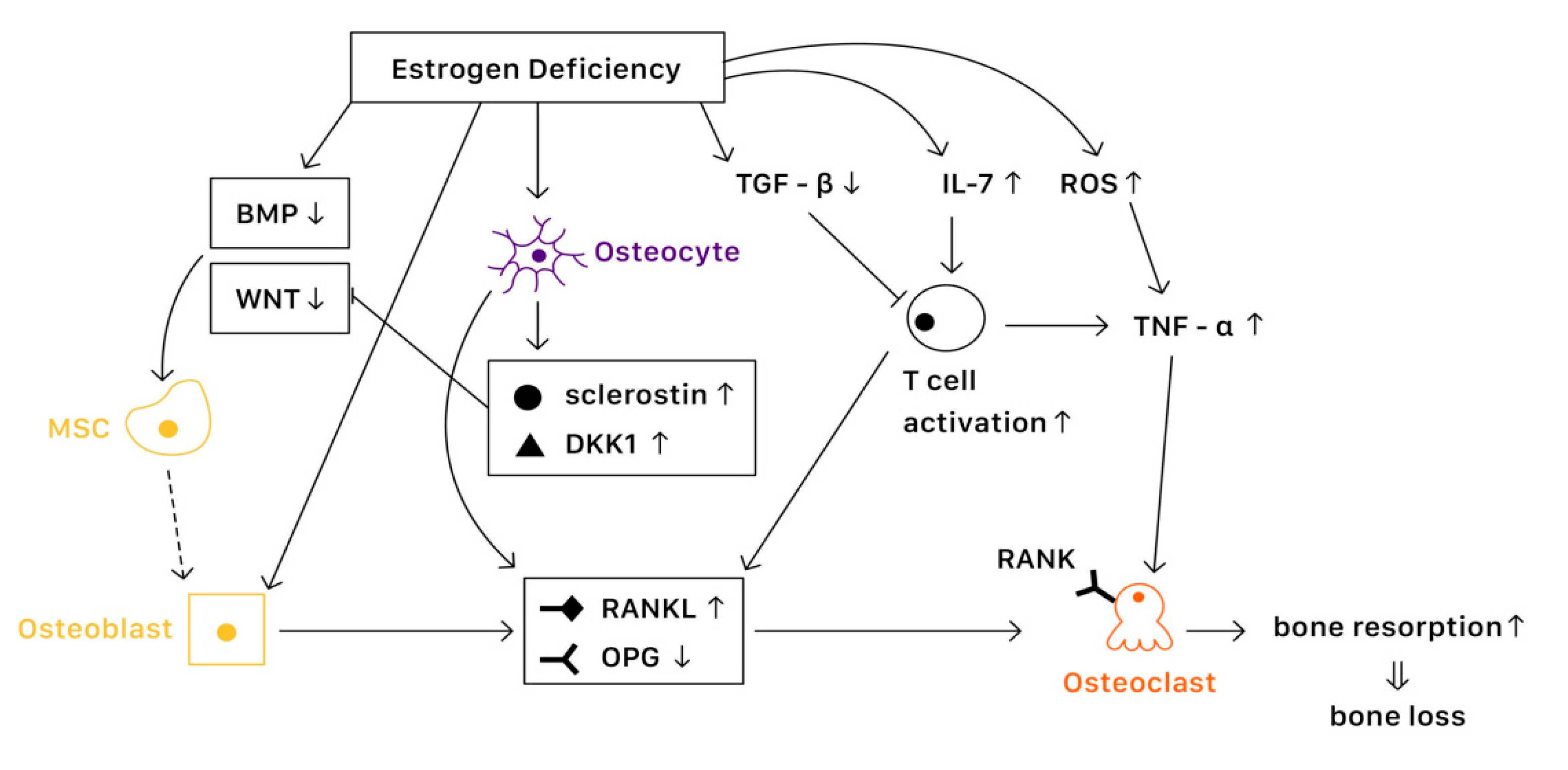
Figure 1. The mechanisms of estrogen deficiency related osteoporosis (EDOP).
1.1. Estrogen Signaling
Estrogen binds with estrogen receptors (ERs), which regulate the expression of estrogen target genes-encoding proteins such as IL-1, insulin-like growth factor 1 (IGF1), and TGFβ [19][1]. Furthermore, ERs can also suppress the action of nuclear factor-κβ ligand (RANKL), thus inhibiting osteoclast formation and bone resorptive activity [20][2]. The lack of estrogen will alter the expression of estrogen target genes, increasing the secretion of IL-1, IL-6, and tumor necrosis factor (TNF). Studies have also shown that estrogen deficiency directly affects cell differentiation and apoptosis [21][3]. The net effects of estrogen deficiency are increased bone turnover and enhanced bone resorption, which result in osteoporosis.
1.2. In Osteoblasts
Several pathways are essential to the activation of osteoblast and bone formation, such as canonical WNT, bone morphogenetic protein (BMP), and TGFβ signaling pathway. Almeida et al. demonstrated that ER complex in osteoblast progenitors activated Wnt/β-catenin signaling, thereby increasing osteogenesis [22][4]. Estrogen is also known to upregulate BMP signaling, which promotes mesenchymal stem cell differentiation from pre-osteoblasts to osteoblasts, rather than adipocytes. Moreover, estrogens stimulate the production of IGF1 and TGFβ by osteoblasts, enhancing bone formation [19][1].
1.3. In Osteoclasts
Various factors are involved in the differentiation and activation of osteoclasts. One key pathway is the NF-κB signaling. The receptor activator of NF-κB (RANK) is expressed on osteoclasts, which is activated when binding with the receptor activator of NF-κB ligand (RANKL) and suppressed when binding with osteoprotegerin (OPG) [23][5]. Estrogen regulates RANKL and OPG, promoting the expression of OPG and thereby reducing bone resorption. Moreover, estrogen inhibits osteoclast differentiation and advocates osteoclast apoptosis by increasing the production of TGFβ [19][1]. In the status of estrogen deficiency, RANKL expression is induced, which leads to osteoclastogenesis.
1.4. In Osteocytes
Osteocytes serve as mechanosensors and control bone remodeling and mineralization. Lee et al. revealed that in the absence of ERα and its complex, osteocytes were unable to provoke adequate response to mechanical strain, indicating that estrogen deficiency was associated with the impairment of mechanosensors in osteocytes [24][6]. On the other hand, osteocytes also produce RANKL, which activates osteoclast formation. Additionally, osteocytes inhibit Wnt signaling by forming sclerostin that can bind with Wnt co-receptor LRP5/6, therefore reduce bone formation [23][5]. In contrast, estrogen retrains the production of sclerostin, protecting bone stability.
1.5. Immune Response
Estrogen deficiency leads to the increase of IL-7 to promote the activation of T cells, which induce pro-inflammatory molecules such as IL-1, IL-6, and TNFα, resulting in osteoclast formation [25,26][7][8]. Moreover, estrogen deficiency also amplifies T cell activation and osteoclastogenesis by increasing reactive oxygen species (ROS), leading to the production of TNF [26][8]. Furthermore, RANKL levels are also upregulated in mesenchymal stem cells (MSCs) and T cells as well as in B cells under a lack of estrogen, causing osteoporosis [19][1].
2. Risk Factor
2.1. Bone Mineral Density
A change in bone mineral density (BMD) is an important predictor for osteoporosis and subsequent fracture. In general, lower BMD is related to a higher risk of fracture. It is estimated that decreasing 1 standard deviation of BMD increases the risk of spine and hip fracture 2.3 to 2.6 fold [27][9]. Fracture risk can be estimated by Kanis algorithm, which is a model based on BMD, age, and fracture accidents. One cohort study suggested that it can be used in healthy females at menopause. However, underestimation of absolute fracture risks in the Kanis algorithm has been noted [28][10]. Bone turnover markers are products released during a bone remodeling process, and they correlate with lumbar spine BMD, providing a possible evaluation for the osteoporosis and fracture risks [29][11].
2.2. Genetics
Genetic factors play an important role in osteoporosis. Several single nucleotide polymorphisms (SNPs), such as estrogen receptor α gene (ESR1) and major histocompatibility complex gene (MHC), were associated with age of natural menopause and possible subsequent osteoporosis [30][12]. Another study revealed that in postmenopausal females, long non-coding RNA (lncRNA) small nucleolar RNA host gene 1 (SNHG1) was down-regulated. An even lower expression level was noted in the postmenopausal osteoporosis group, which might serve as a biomarker for postmenopausal osteoporosis in the future [31][13].
2.3. Menopause Status
Low levels of circulating estrogen are associated with the risk of postmenopausal osteoporosis. Several studies have shown that time since menopause appears to have an association with postmenopausal osteopenia and osteoporosis [32,33,34][14][15][16]. Sioka et al. demonstrated that early menopause between 40 and 45 years of age is correlated with low BMD in postmenopausal females [35][17]. Svejme et al. found an increased risk of osteoporosis, fragility fractures, and mortality in females with menopause before age 47 [36][18]. Furthermore, cumulative exposure to estrogen is a protective against osteoporosis. Parker et al. revealed an increased incidence of osteoporosis in females with less than 25 years of menstruation [37][19]. Due to the fact that premature ovarian insufficiency and early menopause are closely related to osteoporosis, raising concerns and filling up knowledge gaps among the public are needed [38][20].
2.4. Body Weight
Low body weight is a well-documented risk factor for osteoporosis, whereas overweightness is a protective factor [39][21]. Females with a body mass index (BMI) lower than 20 kg/m2 have a significantly higher risk ratio for osteoporosis. On the contrary, for females with a BMI between 25 kg/m2 and 35 kg/m2, the difference in risk ratio appears smaller [27][9]. Moreover, another study has suggested that not only body mass, but also the body composition components and segmental distribution of mass in the trunk and leg are important for the prevention of osteoporosis [40][22].
2.5. Lifestyle
Multiple lifestyle factors are associated with a higher osteoporosis risk. Alcohol intake of more than two units daily, cigarette smoking, poor nutrition status, and lack of physical activities are risk factors of osteoporosis. Interestingly, longer sleep durations as well as longer daytime napping times tend to present higher risks of osteoporosis, which could be explained by the reduction of weight-bearing physical activities due to extended sleeping time [41][23].
2.6. Secondary Causes
Various medications and chronic diseases are related to bone loss. There is strong evidence that glucocorticoids are associated with osteoporosis, especially in populations with long term use. In addition, studies have revealed that osteoporosis was found in more than 50% of postmenopausal females and males with rheumatic arthritis. Possible mechanisms for this finding included inflammatory processes and cumulative high glucocorticoid doses [42][24]. Early menopause due to oncological diseases is also a risk factor of osteoporosis. Oncological treatments, such as surgical, pharmacological, or radiological therapies are associated with iatrogenic menopause and subsequent osteoporosis [43][25]. Compared with natural menopause, surgical menopause is found to be associated with lower BMD and higher rates of osteoporosis [44,45][26][27].
3. Management
3.1. Hormone Therapy
Systemic hormone therapy has shown favorable benefits over harm in females under the age of 60 years or up to 10 years after menopause. For symptomatic postmenopausal females, hormone therapy is a reasonable choice for symptom relief and prevention of bone loss [46][28]. In females who have undergone hysterectomy, estrogen is given alone. To decrease the risk of endometrial hyperplasia and carcinoma with estrogen use, progestogens and the selective estrogen receptor modulator (SERM) are added into estrogen-base hormone therapy for females with an intact uterus [47][29]. A novel way of excision of ovarian tissues in the youth and cryostorage for the use after menopause have been discussed. Grafted tissues serve endocrine functions which prevent osteoporosis and menopause-related conditions. Nevertheless, further studies are required for to determine feasibility [48][30].
3.2. Selective Estrogen-Receptor Modulators
Selective estrogen receptor modulators (SERMs) are a class of drugs that can act on estrogen receptors, which act as estrogen agonists or antagonists in different tissues. Raloxifene is the first SERM approved for the treatment of postmenopausal osteoporosis, lowering the risk of vertebral fractures [49][31]. Bazedoxifene (BZA) is a third-generation SERM with high affinity for the estrogen receptor (ER) alpha. BZA has been combined with conjugated equine estrogen (CEE) to create a tissue selective estrogen complex (TSEC) for the management of vasomotor symptoms (VMS) and the prevention of osteoporosis (OP) associated with menopause [50][32]. Trials revealed that TSEC has a benefit on improving vulvo-vaginal atrophy, reducing hot flashes and preventing bone loss. There is also a good safety and tolerability profile [51][33]. TSEC has been approved by the U.S. Food and Drug Administration in 2013 based on five phase 3 studies known as the Selective estrogens, Menopause And Response to Therapy (SMART) trials. For prevention of osteoporosis, the approved dose of BZA/CEE is 20 mg BZA and 0.45 mg CEE [52][34].
3.3. Bisphosphonates
Bisphosphonates, including alendronate, risedronate, and zoledronic acid, are valuable first-line agents of choice in the treatment of postmenopausal osteoporosis [46][28]. Clinical trials have proven that bisphosphonate increases BMD at hip and vertebral sites significantly in postmenopausal females, reducing fracture risks [27][9]. A common adverse effect of bisphosphonates is gastrointestinal upset. Despite being widely used, oral bisphosphonates must be administrated with caution in patients with a history of gastroesophageal reflux diseases, Barrett’s esophagus, or peptic ulcers [49][31].
3.4. Calcium and Vitamin D
It is known that many females do not have sufficient calcium and vitamin D intake. Therefore, adequate calcium and vitamin D supplementation is important for building up healthy bone structures in order to prevent osteoporosis. For postmenopausal females, a total daily intake of 1200 mg of calcium and daily supplementation with 800 to 2000 IU of vitamin D are recommended to improve BMD [46][28].
3.5. Parathyroid Hormone (Teriparatide)
Treatment with teriparatide should be considered in postmenopausal females with severe osteoporosis who cannot tolerate other treatments or those who are experiencing new fractures in spite of antiresorptive therapy [46][28]. Contraindications for teriparatide usage include patients with risks of osteosarcoma, Paget’s disease, hypercalcemia, prior radiation therapy, or a history of previous bone malignancies [49][31].
3.6. RANKL Inhibitor
Denosumab, one of the RANKL inhibitors, is a highly effective and safe treatment for patients with postmenopausal osteoporosis, reducing the risk of vertebral, non-vertebral, and hip fractures [46][28]. Denosumab is injected every 6 months via a subcutaneous route, which increases compliance, and thus is suggested in patients who cannot tolerate or have failed other treatments. For RANKL inhibitor users, calcium levels should be monitored in patients predisposed to hypocalcemia. Skin infection has been reported as a more commonly noted side effect [27][9].
3.7. Lifestyle Modification
A balanced diet, adequate physical activities, cessation of smoking, and limited alcohol consumption are recommended for osteoporosis prevention [53][35]. Protein restriction is associated with muscle and bone loss, increasing fragility in bone [54][36]. An in vivo study demonstrated that olive oil had the property of anti-osteoporosis with an increase in BMD [55][37]. Therefore, a balanced diet is important for the prevention of osteoporosis. Adequate physical activities have a positive effect on bone health, muscle strength, and balance, which can lower the risk of falling and fractures [56][38]. In females with fair functional capacity, anaerobic exercise that combines resistance with weight bearing is recommended as well as aerobic exercise. Especially, exercise that focuses on balance should be considered for females at risk of falls [49][31].
References
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal Osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069.
- Abu-Amer, Y. NF-ΚB Signaling and Bone Resorption. Osteoporos Int. 2013, 24, 2377–2386.
- Riggs, B.L. The Mechanisms of Estrogen Regulation of Bone Resorption. J. Clin. Investig. 2000, 106, 1203–1204.
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen Receptor-α Signaling in Osteoblast Progenitors Stimulates Cortical Bone Accrual. J. Clin. Investig. 2013, 123, 394–404.
- Bado, I.; Gugala, Z.; Fuqua, S.A.W.; Zhang, X.H.-F. Estrogen Receptors in Breast and Bone: From Virtue of Remodeling to Vileness of Metastasis. Oncogene 2017, 36, 4527–4537.
- Lee, K.; Jessop, H.; Suswillo, R.; Zaman, G.; Lanyon, L. Endocrinology: Bone Adaptation Requires Oestrogen Receptor-Alpha. Nature 2003, 424, 389.
- McLean, R.R. Proinflammatory Cytokines and Osteoporosis. Curr. Osteoporos Rep. 2009, 7, 134–139.
- Weitzmann, M.N.; Pacifici, R. Estrogen Deficiency and Bone Loss: An Inflammatory Tale. J. Clin. Investig. 2006, 116, 1186–1194.
- Management of Osteoporosis in Postmenopausal Women: 2010 Position Statement of The North American Menopause Society. Menopause 2010, 17, 25–54.
- Abrahamsen, B.; Vestergaard, P.; Rud, B.; Bärenholdt, O.; Jensen, J.-E.B.; Nielsen, S.P.; Mosekilde, L.; Brixen, K. Ten-Year Absolute Risk of Osteoporotic Fractures According to BMD T Score at Menopause: The Danish Osteoporosis Prevention Study. J. Bone Miner. Res. 2006, 21, 796–800.
- Gurban, C.V.; Balaş, M.O.; Vlad, M.M.; Caraba, A.E.; Jianu, A.M.; Bernad, E.S.; Borza, C.; Bănicioiu-Covei, S.; Motoc, A.G.M. Bone Turnover Markers in Postmenopausal Osteoporosis and Their Correlation with Bone Mineral Density and Menopause Duration. Rom. J. Morphol. Embryol. 2019, 60, 1127–1135.
- Zhao, L.; Cui, B.; Liu, J.; Zhang, M.; Zhao, H.; Sun, L.; Tao, B.; Zhang, L.; Ning, G. Interactions of Osteoporosis Candidate Genes for Age at Menarche, Age at Natural Menopause, and Maximal Height in Han Chinese Women. Menopause 2011, 18, 1018–1025.
- Huang, S.; Zhu, X.; Xiao, D.; Zhuang, J.; Liang, G.; Liang, C.; Zheng, X.; Ke, Y.; Chang, Y. LncRNA SNHG1 Was Down-Regulated after Menopause and Participates in Postmenopausal Osteoporosis. Biosci. Rep. 2019, 39, BSR20190445.
- Fistarol, M.; Rezende, C.R.; Figueiredo Campos, A.L.; Kakehasi, A.M.; Geber, S. Time since Menopause, but Not Age, Is Associated with Increased Risk of Osteoporosis. Climacteric 2019, 22, 523–526.
- Qiu, C.; Chen, H.; Wen, J.; Zhu, P.; Lin, F.; Huang, B.; Wu, P.; Lin, Q.; Lin, Y.; Rao, H.; et al. Associations between Age at Menarche and Menopause with Cardiovascular Disease, Diabetes, and Osteoporosis in Chinese Women. J. Clin. Endocrinol. Metab. 2013, 98, 1612–1621.
- Yoldemir, T.; Erenus, M.; Durmusoglu, F. The Impact of Serum FSH and Estradiol on Postmenopausal Osteoporosis Related to Time since Menopause. Gynecol. Endocrinol. 2012, 28, 884–888.
- Sioka, C.; Fotopoulos, A.; Georgiou, A.; Xourgia, X.; Papadopoulos, A.; Kalef-Ezra, J.A. Age at Menarche, Age at Menopause and Duration of Fertility as Risk Factors for Osteoporosis. Climacteric 2010, 13, 63–71.
- Svejme, O.; Ahlborg, H.G.; Nilsson, J.-Å.; Karlsson, M.K. Early Menopause and Risk of Osteoporosis, Fracture and Mortality: A 34-Year Prospective Observational Study in 390 Women. BJOG 2012, 119, 810–816.
- Parker, S.E.; Troisi, R.; Wise, L.A.; Palmer, J.R.; Titus-Ernstoff, L.; Strohsnitter, W.C.; Hatch, E.E. Menarche, Menopause, Years of Menstruation, and the Incidence of Osteoporosis: The Influence of Prenatal Exposure to Diethylstilbestrol. J. Clin. Endocrinol. Metab. 2014, 99, 594–601.
- Goh, M.; Nguyen, H.H.; Khan, N.N.; Milat, F.; Boyle, J.A.; Vincent, A.J. Identifying and Addressing Osteoporosis Knowledge Gaps in Women with Premature Ovarian Insufficiency and Early Menopause: A Mixed-Methods Study. Clin. Endocrinol. 2019, 91, 498–507.
- Akdeniz, N.; Akpolat, V.; Kale, A.; Erdemoglu, M.; Kuyumcuoglu, U.; Celik, Y. Risk Factors for Postmenopausal Osteoporosis: Anthropometric Measurements, Age, Age at Menopause and the Time Elapsed after Menopause Onset. Gynecol. Endocrinol. 2009, 25, 125–129.
- Liu, S.; Li, J.; Sheng, Z.; Wu, X.; Liao, E. Relationship between Body Composition and Age, Menopause and Its Effects on Bone Mineral Density at Segmental Regions in Central Southern Chinese Postmenopausal Elderly Women with and without Osteoporosis. Arch. Gerontol. Geriatr. 2011, 53, e192–e197.
- Chen, G.; Chen, L.; Wen, J.; Yao, J.; Li, L.; Lin, L.; Tang, K.; Huang, H.; Liang, J.; Lin, W.; et al. Associations between Sleep Duration, Daytime Nap Duration, and Osteoporosis Vary by Sex, Menopause, and Sleep Quality. J. Clin. Endocrinol. Metab. 2014, 99, 2869–2877.
- Oelzner, P.; Schwabe, A.; Lehmann, G.; Eidner, T.; Franke, S.; Wolf, G.; Hein, G. Significance of Risk Factors for Osteoporosis Is Dependent on Gender and Menopause in Rheumatoid Arthritis. Rheumatol. Int. 2008, 28, 1143–1150.
- Baldi, S.; Becorpi, A. Prevention, Diagnosis and Treatment of Osteoporosis Following Menopause Induced Due to Oncological Disease. Clin. Cases Miner. Bone Metab. 2009, 6, 261–263.
- Özkaya, E.; Cakir, E.; Okuyan, E.; Cakir, C.; Ustün, G.; Küçüközkan, T. Comparison of the Effects of Surgical and Natural Menopause on Carotid Intima Media Thickness, Osteoporosis, and Homocysteine Levels. Menopause 2011, 18, 73–76.
- Ozdemir, S.; Celik, C.; Görkemli, H.; Kiyici, A.; Kaya, B. Compared Effects of Surgical and Natural Menopause on Climacteric Symptoms, Osteoporosis, and Metabolic Syndrome. Int. J. Gynaecol. Obstet. 2009, 106, 57–61.
- Khan, A.; Fortier, M.; Menopause and Osteoporosis Working Group. Osteoporosis in Menopause. J. Obstet. Gynaecol. Can. 2014, 36, 839–840.
- Rees, M.; Angioli, R.; Coleman, R.L.; Glasspool, R.; Plotti, F.; Simoncini, T.; Terranova, C. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) Position Statement on Managing the Menopause after Gynecological Cancer: Focus on Menopausal Symptoms and Osteoporosis. Maturitas 2020, 134, 56–61.
- Andersen, C.Y.; Kristensen, S.G. Novel Use of the Ovarian Follicular Pool to Postpone Menopause and Delay Osteoporosis. Reprod. Biomed. Online 2015, 31, 128–131.
- Mendoza, N.; Sánchez-Borrego, R.; Villero, J.; Baró, F.; Calaf, J.; Cancelo, M.J.; Coronado, P.; Estévez, A.; Fernández-Moya, J.M.; González, S.; et al. 2013 Up-Date of the Consensus Statement of the Spanish Menopause Society on Postmenopausal Osteoporosis. Maturitas 2013, 76, 99–107.
- Palacios, S.; Mejía Ríos, A. Bazedoxifene/Conjugated Estrogens Combination for the Treatment of the Vasomotor Symptoms Associated with Menopause and for Prevention of Osteoporosis in Postmenopausal Women. Drugs Today 2015, 51, 107–116.
- Tella, S.H.; Gallagher, J.C. Bazedoxifene + Conjugated Estrogens in HT for the Prevention of Osteoporosis and Treatment of Vasomotor Symptoms Associated with the Menopause. Expert Opin. Pharmacother. 2013, 14, 2407–2420.
- Umland, E.M.; Karel, L.; Santoro, N. Bazedoxifene and Conjugated Equine Estrogen: A Combination Product for the Management of Vasomotor Symptoms and Osteoporosis Prevention Associated with Menopause. Pharmacotherapy 2016, 36, 548–561.
- Ring, M. Women’s Health: Polycystic Ovarian Syndrome, Menopause, and Osteoporosis. Prim. Care 2017, 44, 377–398.
- de Quadros, V.P.; Tobar, N.; Viana, L.R.; Dos Santos, R.W.; Kiyataka, P.H.M.; Gomes-Marcondes, M.C.C. The 17β-Oestradiol Treatment Minimizes the Adverse Effects of Protein Restriction on Bone Parameters in Ovariectomized Wistar Rats: Relevance to Osteoporosis and the Menopause. Bone Jt. Res. 2019, 8, 573–581.
- Liu, H.; Huang, H.; Li, B.; Wu, D.; Wang, F.; Zheng, X.H.; Chen, Q.; Wu, B.; Fan, X. Olive Oil in the Prevention and Treatment of Osteoporosis after Artificial Menopause. Clin. Interv. Aging 2014, 9, 2087–2095.
- Eisenberg Center at Oregon Health & Science University. Osteoporosis Treatments That Help Prevent Broken Bones: A Guide for Women After Menopause. In Comparative Effectiveness Review Summary Guides for Consumers; AHRQ Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2005.
More
