1. Introduction
An increasingly aging population is now a global phenomenon, especially in developed countries
[1]. Thus, interventions are needed to ameliorate physical fitness-related changes to guarantee higher functional capacity, autonomy, and health among elderly people.
Osteoporosis is a vital healthcare issue among the elderly people. During the aging process, a gradual loss of bone mass results in osteopenia and osteoporosis. Both sexes are affected; however, the main burden of disease falls upon menopausal women. Osteoporosis substantially increases the risk of skeletal fractures and further morbidity and mortality
[2,3][2][3]. The cumulative lifetime fracture risk for a 50-year old woman with osteoporosis can be as high as 60%
[4]. Effective fracture prevention, if achievable by reducing the loss of bone mass, will have a major impact on an individual’s morbidity and, to a lesser extent, mortality
[2].
The diagnostic difference between osteopenia and osteoporosis is based on the level of bone mineral density (BMD). The World Health Organization (WHO) recommends measuring BMD at the spine, hip, or forearm using dual-energy X-ray absorptiometry devices
[5]. According to the WHO criteria, osteopenia is defined as a BMD between 1.0 and 2.5 SD below that of a “young normal” adult (T score between −1.0 and −2.5), and osteoporosis as a T score of −2.5 or lower.
Some people may also have a congenitally lower bone density. Childhood and adolescence are important stages in optimal bone formation and thus the prevention of osteoporosis in older age. Although heritable factors account for 60–80% of optimal bone mineralization, modifiable factors such as weight-bearing exercise, nutrition, body mass, and hormonal milieu affect the development of osteopenia and osteoporosis in adulthood
[1].
A primary goal in the screening and treating of osteoporosis is to prevent the development of subsequent osteoporotic fractures. Effective fracture prevention will have a major impact on an individual’s morbidity and mortality
[2]. BMD changes following treatment might also provide a better prediction of the anti-fracture efficacy of a therapeutic agent.
At a molecular level, bone is predominantly composed of the type 1 collagen matrix, which is strengthened with the apposition of calcium hydroxyapatite crystals
[6]. Osteoblasts are derived from bone marrow stromal cells (BMSCs), which are self-renewing in vivo. Osteoclasts are multi- nucleated giant cells that resorb the bone matrix, ensuring development and continuous remodeling of the skeleton and the bone marrow hematopoietic niche
[7]. Osteocytes are the most numerous, longest-lived cells of bone. They are osteoblasts that become entombed in lacunae in the bone matrix (osteoid) that they synthesize
[6]. These osteoblasts undergo a morphologic change and become osteocytes with cytoplasmic processes that connect them with other osteocytes and flattened lining cells
[6].
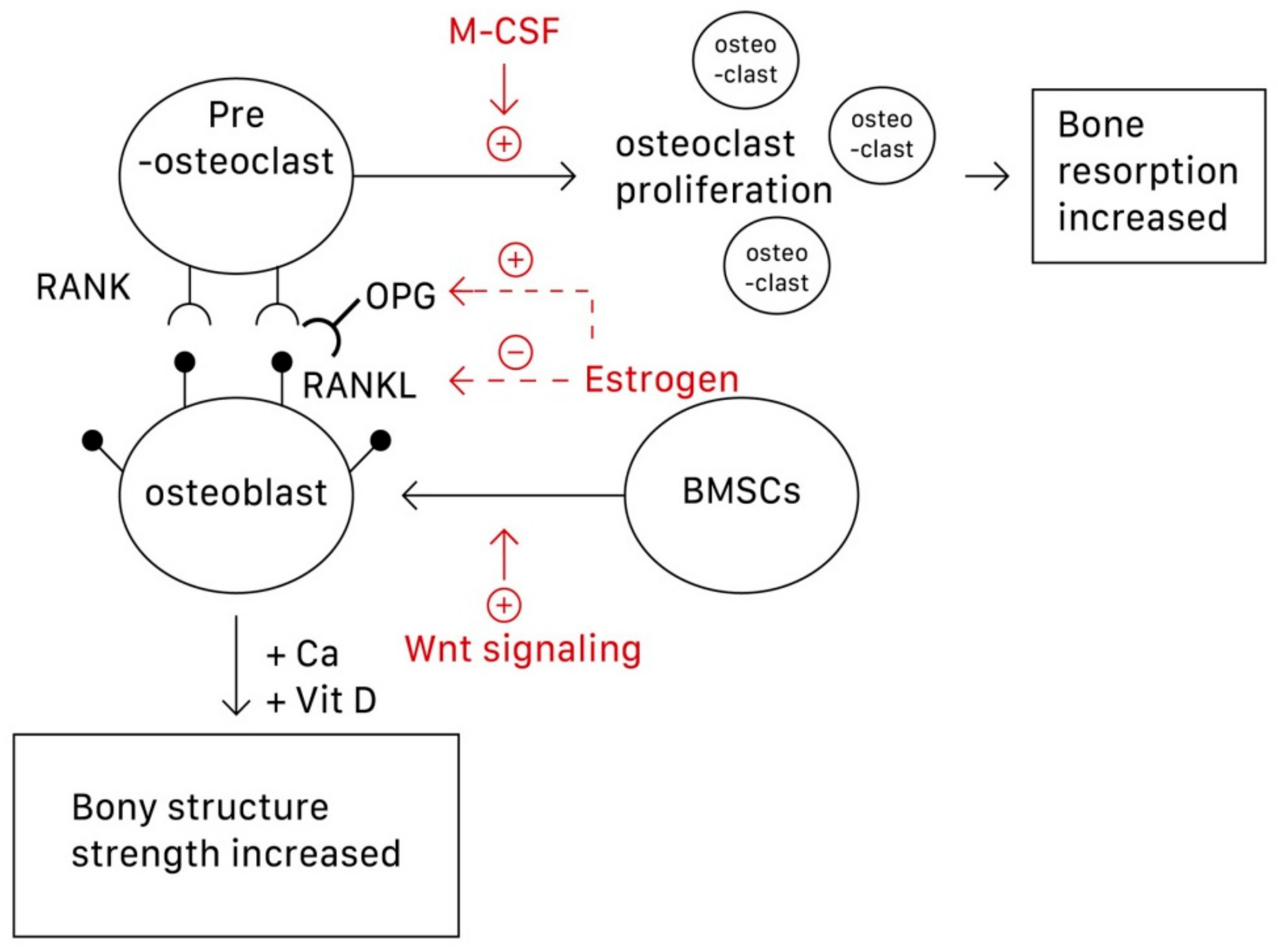
Although osteoclasts are the main focus of osteoporosis therapy, osteoblasts have recently been established as potential targets
[8]. Specifically, the interactions among the RANK/RANKL (receptor activator of nuclear factor-κB (NF-κB) ligand)/OPG (osteoprotegerin) system, Wnt/β-catenin signaling, and interleukin within osteoblasts and osteoclasts construct the bone remodeling cycle
[8,9,10][8][9][10]. This is because osteoblasts can produce both RANKL and OPG. The function of OPG is to prevent RANKL from binding to RANK, which lies on the surface of osteoclasts and activates the proliferation of osteoclasts and bone resorption, thus inhibiting osteoclastogenesis. Wnt signaling regulates bone homeostasis by stimulating osteoblast differentiation and function and inhibiting osteoclast differentiation, mostly through indirect mechanisms via osteoblasts
[8]. Wnt signaling can be classified into noncanonical (β-catenin-independent) and canonical (β-catenin-dependent) signaling pathways. In the latter, β-catenin accumulates in the cytoplasm and engages the nuclear area to express bone formation genes such as OPG and RANKL
[9]. Estrogen is perhaps the most direct and effective approach to preventing and treating osteoporosis because it inhibits osteoclastic bone resorption. Osteoclasts require activation by two cytokines, namely M-CSF and RANKL, which are produced by BMSCs and osteoblasts, respectively. Estrogen suppresses osteoclasts by reducing the expression of RANKL in marrow cells and increases OPG secretion by osteoblasts, which inactivates RANK
[10]. Estrogen thus decreases the secretion of RANKL and inactivates RANKL mediating osteoclastogenesis. In postmenopausal women, the estrogen level in the body decreases, dysregulating the RANKL/RANK/OPG system, and osteoclastogenesis then exceeds osteoblastogenesis
[10].
Figure 1 shows the pathways underlying the actions of osteoblasts, osteoclasts, and subsequent osteoporosis.
Figure 1. The pathways underlying the formation and action of osteoblasts, osteoclasts, and subsequent osteoporosis. M-CSF can promote the proliferation of osteoclasts; Wnt signaling can stimulate the differentiation of BMSCs into osteoblasts. Estrogen can both increase the secretion of OPG and decrease secretion of RANKL, thus preventing the combination of RANKL-RANK to activate osteoclastogenesis. BMSCs, bone marrow stromal cells; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κB (NF-κB) ligand).
Nutritional support, such as adequate calcium and vitamin D, as well as exercise are among the crucial cornerstones of the prevention of osteoporosis and are necessary during treatment with pharmacologic agents
[1,11,12,13,14][1][11][12][13][14]. However, current evidence has shown that routine calcium and vitamin D supplements in healthy individuals are not needed. In patients with an adequate vitamin D status and a normal calcium intake, routine calcium and vitamin D supplements are not required in most people receiving treatment for osteoporosis, where routine use has not been shown to affect treatment efficacy
[15,16][15][16]. Several new therapeutic agents are now available for the treatment of osteoporosis
[3]. Although most have been found to significantly reduce the occurrence of vertebral fractures, controversy remains regarding the evidence for their nonvertebral or hip anti-fracture effects
[1,3,8][1][3][8]. Nevertheless, physical modalities have been shown to have some beneficial effects in people with osteoporosis
[17].
2. Calcium and Vitamin D Supplementation
2.1. Calcium
Calcium is a vital element in human physiology. It plays a key role in muscle contraction, bone strength, nerve impulses and transmission, regulation of the heartbeat, and fluid balance within cells
[1]. With regard to calcium deposition, calcium hydroxyapatite crystals add into the predominant type I collagen matrix in bone and increases bone strength
[6]. By contrast, insufficient calcium intake causes a suboptimal bone mass peak and low bone mineralization, possibly leading to osteoporosis and fractures.
Because peak adult bone mass is achieved between age 30 and 35 years, elderly people and adolescents must maintain a higher calcium intake
[12]. However, it is impossible to accurately identify the oral calcium amount that needs to be ingested as numerous factors affect the intestinal absorption of calcium, including age, sex, gonadal function, ethnic group, and the pattern of calcium intake.
Although calcium is physiologically necessary, a recent systematic review has shown that people in several Asian countries have an average dietary calcium intake of less than 500 mg/day
[13]. People in African and South American countries also have a low calcium intake between 400 and 700 mg/day. Only people in Northern European countries have a calcium intake greater than 1000 mg/day
[13]. The National Health and Nutrition Examination Survey 2003–2006 data in the United States show that dietary supplements provide an adequate intake of calcium for only 9% of the whole population
[14].
According to the calcium allowances recommended by the WHO, based on North American and Western European data and the current recommendations of the European Union, Australia, Canada, United States, and the United Kingdom, an adequate intake of calcium for children aged seven to nine is approximately 400–700 mg per day. For pubertal and adolescent boys and girls, the recommended calcium intake is approximately 800–1300 mg per day. For men aged 19–65 and women from 19 years of age to the menopause, approximately 800–1000 mg per day of elemental calcium is recommended
[8,12][8][12]. The National Osteoporosis Foundation advises men above 65 years and postmenopausal women to consume at least 1200 mg per day of elemental calcium
[8]. As a treatment for osteoporosis, the Committee on Medical Aspects of Foot and Nutrition Policy (COMA) recommends taking more than 700 mg of calcium per day to maintain bone health.
The role played by calcium in reducing the risk of fractures is controversial. A nine-year follow-up of a prospective cohort study in Korea, involving 2158 men and 2153 women aged over 50, evaluated the association between a higher dietary calcium intake and the risk of fracture. The result showed that increased dietary calcium intake was not associated with any reduction in the risk of fracture among Korean women
[18]. A systematic review of two randomized controlled trials (RCTs) and 44 cohort studies in 2015 concluded that increasing calcium intake from dietary sources cannot prevent fractures. In four RCTs (
n = 44,505), the evidence showed that the effect of calcium supplements in preventing fractures is weak and inconsistent
[19]. The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Osteoporosis Foundation also concluded that current evidence reveals that supplementation with calcium alone cannot reduce the risk of fracture
[12].
2.2. Vitamin D
Vitamin D is also an important factor in maintaining bone health. Following exposure of the skin to sunlight and UV B radiation, vitamin D3 is synthesized and subsequently converted into 25 hydroxyvitamin D in the liver by the enzyme 25-hydroxylase. It is then transported to the kidney, where it becomes 1,25-dihydroxycholecalciferol (also known as calcitriol), the active form of vitamin D in the human body
[1]. Vitamin D can enhance the intestinal absorption of calcium, and it interacts with the parathyroid hormone to help maintain calcium homeostasis between the blood and bones. It can also regulate bone remodeling via binding with the vitamin D receptor (VDR) and initiating transcription of a VDR-associated gene. However, the direct effect of vitamin D on osteocytes remains unclear.
The dietary sources of vitamin D include salt-water oily fish and liver, egg yolks, margarine, some yogurts, cheeses, cereals, and vitamin D-fortified milk and orange juice. However, the major source of vitamin D3 for most humans is synthesis in the skin under the influence of UV light
[1,15][1][15]. The prevalence of vitamin D deficiency has been reported to be higher in the elderly population. This might be due to insufficient skin exposure to sunlight and reduced efficacy of vitamin D synthesis. Indoor styles of living and clothing now result in low sun exposure
[15,20][15][20]. UV irradiation, half the minimal erythematous dose, on 1000 cm
2 of skin on the backs of elderly participants three times per week was found to be as effective as the oral intake of 400 IU vitamin D
[20]. According to previous studies, the recommended daily oral dosage of vitamin D ranges from 400 to 2000 IU
[1,8][1][8]. In a report by the National Osteoporosis Foundation, people older than 50 years are recommended to take at least 800–1000 IU of vitamin D daily because they are at greater risk of vitamin D deficiency
[21]. However, a recent review revealed that supplements are better targeted based on clinical status to frail older people and possibly to people with dark skin living at higher latitudes
[15]. Recent trials showed that vitamin D increases bone density when 25-hydroxyvitamin D levels are below 25–30 nmol/L. A daily dose of 400–800 IU (10–20 μg) is usually adequate
[15]. Recent evidence has revealed that low dose vitamin D is safe, but high doses result in more falls and fractures
[15,16][15][16]. Current evidence does not support the use of these supplements in healthy community-dwelling adults
[15,16][15][16]. Vitamin D intoxication is one of the medical conditions and is typically observed when taking >10,000 IU of vitamin D per day for more than 5 months
[22,23][22][23]. People who are at risk for hypercalciuria and hypercalcemia should monitor their 25(OH) D concentration more frequently.
However, whether direct vitamin D supplementation can increase BMD remains controversial. In 2019, a RCT was conducted to treat 400 people between 30 and 60 years of age for vitamin D deficiency with 50,000 IU per week for 8 weeks. The results showed that the prevalence of osteoporosis in the intervention group was significantly lower than that in the control group
[24]. However, another prospective, randomized, double-blind, placebo-controlled, three-year clinical trial of vitamin D3 supplementation in 260 postmenopausal African American women showed no significant difference in BMD between placebo and vitamin D3 groups, although participants in the intervention group performed better in terms of gait speed and grip strength
[25]. Moreover, another RCT published in 2019, comprising a three-year follow-up of 311 healthy men and women aged 55–70 years, concluded that a greater loss of BMD occurred with a higher intake of vitamin D (4000 IU per day or 10,000 IU per day, compared with 400 IU per day). Furthermore, no significant difference between the three groups in terms of bone strength was found
[26]. The conflicting results and discrepancy among these three studies may be due to different age and race of participants, different dosage of vitamin D and study design
[24,25,26][24][25][26]. In order to minimize study heterogeneity in future research,
the weresearchers urge standardization of the many variables involved in trials, such as age of participants and time of usage that may have crucial effect on the response. Dosage of vitamin D should be consistent across trials for better communication and prediction of therapeutic activity. Finally, bigger sample sizes are needed to increase the validity and generalizability of results.
2.3. Combined Use of Calcium and Vitamin D
Regarding the supplementation of calcium and vitamin D, an increase in dietary calcium should be considered first. When adequate dietary calcium intake cannot be achieved, calcium supplement tablets could be used
[1]. Calcium carbonate is bioavailable when taken with a meal. Calcium citrate is recommended for individuals with a history of renal stones.
The best sources of calcium are dairy products including milk, yogurt and cheese. Calcium is also found in nuts, dark-green leafy vegetables, dried peas and beans. Vitamin D occurs naturally in only a few foods (salt-water oily fish, egg yolks, and liver,) and is also available in fortified foods (milk and milk products such as yogurts, cheeses and margarines), some juices, and breakfast cereals
[14]. While the dietary sources of vitamin D are often insufficient to meet daily requirement, skin exposure to sunlight may be considered to provide a good resource of vitamin D by synthesis
[20].
Although no consistent results have been found for the benefits of calcium or vitamin D supplements alone, a combination of calcium and vitamin D treatment has been shown to have some benefits in terms of reducing the incidence of fractures
[27,28][27][28]. Several prospective, randomized, placebo-controlled trials have demonstrated the benefits of calcium and vitamin D. The National Osteoporosis Foundation published a meta-analysis in 2016 on calcium plus vitamin D supplementation and fracture prevention
[27]. It included eight RCTs assessing calcium plus vitamin D supplementation versus a placebo on the incidence of total fracture. The enrolled calcium dosage was approximately 500 mg/day in one RCT study and approximately 1000–1200 mg/day in another
[27]. The vitamin D dosage was approximately 800 IU/day in four RCT studies and 400 and 700 IU/day in the remaining two studies. The results showed a significant reduction of 15% in the risk of all fractures and a greater reduction of 30% in the risk of hip fractures
[27]. Another large study in 2010, published by the DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group, recruited seven RCTs of either vitamin D with calcium or vitamin D alone, with fractures as an outcome. This study involved a total of 68,517 participants. The results revealed that vitamin D given alone in doses of 10–20 μg was unable to prevent fractures. However, calcium and vitamin D together led to a decrease in hip fractures and total fractures, and probably also vertebral fractures
[28]. Conversely, the Women’s Health Initiative Calcium and Vitamin D trial (WHI CaD) found no association between combined supplements and height loss in 36,282 healthy postmenopausal women, compared with a placebo. In this study, 1000 mg of elemental calcium in the form of calcium carbonate with 400 IU of daily vitamin D and a placebo were compared
[29]. Recently, the major trials in community-dwelling individuals have also not demonstrated fracture prevention with a combination of calcium and vitamin D
[15,16][15][16]. Nevertheless, the results of a large study in vitamin D-deficient nursing home residents indicated a reduced fracture incidence
[15]. These conflicting results are likely to be due to differences in dosage of supplements, patient populations, and study design
[15,16,28,29][15][16][28][29]. For individuals living in residential institutions, they are more likely to have osteoporosis because of their poorer mobility, infrequent sun exposure, and poorer diet
[16]. For these reasons it is possible that older people living in residential care institutions may benefit from calcium and vitamin D supplements. In summary, benefits of calcium and vitamin D supplementation may differ between people living in the community and people living in residential institutions
[16].
In summary, there is currently no recommendation for the individual use of calcium or vitamin D supplementation to reduce the risk of fracture. However, a combination of calcium and vitamin D supplementation may have some benefits in preventing fractures in the future for specific populations.