Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vincenzo Desiderio and Version 2 by Lindsay Dong.
Soft tissue regeneration holds significant promise for addressing various clinical challenges, ranging from craniofacial and oral tissue defects to blood vessels, muscle, and fibrous tissue regeneration. Mesenchymal stem cells (MSCs) have emerged as a promising tool in regenerative medicine due to their unique characteristics and potential to differentiate into multiple cell lineages.
- mesenchymal stem cells
- regenerative medicine
- tissue regeneration
- tissue engineering
1. Introduction
The repair and restoration of different tissues throughout the human body is a complex and dynamic process, crucial for maintaining the body’s overall integrity and function in response to various injuries and disease states [1][2][1,2]. Soft tissues, including skin, muscles, blood vessels, nerves, and fibrous tissues, play diverse roles and contribute to the body’s integrity and functionality [3]. These tissues are involved in essential functions such as temperature regulation, locomotion, oxygen and nutrient transport, sensory perception, and structural support.
Traditional therapeutic approaches for soft tissue injuries or degenerative disorders have relied on conservative therapies such as physical therapy, medication, and surgery. While these methods offer some benefits like comfort and tissue healing, they often have limitations [4]. Severe soft tissue injuries may surpass the body’s innate regenerative capacity, leading to inadequate repair. Scar tissue formation during healing can impede proper tissue functionality and slow recovery. Furthermore, conventional treatments may struggle to restore tissue structure and function, especially in complex soft tissue environments with diverse cell types and intricate tissue architectures [5].
In recent years, regenerative medicine has emerged as a promising field aiming to overcome the limitations of traditional treatments and provide innovative approaches for soft tissue repair and regeneration [5]. Regenerative medicine is a broad term that encompasses a range of strategies, such as tissue engineering, cellular therapy, gene therapy, and the use of biomaterials, to restore damaged or diseased tissues to their original form and function [6][7][8][6,7,8]. Out of cell-based therapies, mesenchymal stem cells (MSCs) have garnered significant attention due to their unique characteristics and regenerative potential. MSCs possess self-renewal and differentiation capabilities, including the ability of MSCs to differentiate into cell types specific to soft tissues. This ability makes MSCs a particularly promising avenue for soft tissue regeneration [9].
2. Mesenchymal Stem Cells
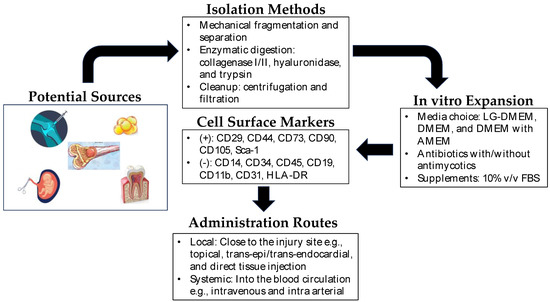
MSCs are a type of multipotent stem cell derived from various sources, such as bone marrow (BM-MSCs), adipose tissue (AD-MSCs), umbilical cord (UC-MSCs), and dental pulp (DP-MSCs) [10]. These cells have gained attention in regenerative medicine due to their ability to self-renew, modulate the immune response, and differentiate into multiple cell lineages. Their differentiation potential into fibroblasts, chondrocytes, osteoblasts, and adipocytes makes them particularly attractive for tissue repair and regeneration [11]. The extraction methods for MSCs vary depending on the tissue source. For example, BM-MSCs are typically harvested from bone marrow aspiration, followed by isolation and expansion in culture. AD-MSCs are obtained through liposuction or surgical excision of adipose tissue, which is then processed to extract the MSCs. UC-MSCs are derived from umbilical cord tissue or blood, while DP-MSCs are obtained from dental pulp extracted for therapeutic or orthodontic purposes [12]. Each source has its advantage and disadvantage as cell yield, proliferation capacity, and differentiation potential, which should be considered based on specific applications. Figure 1 shows the general scheme for the isolation and expansion of MSCs for clinical applications.
Figure 1. Mesenchymal stem cells in regenerative medicine. Depicted above is a brief overview of the isolation, expansion, and administration of mesenchymal stem cells in regenerative therapy. Potential sources of MSCs include synovium, bone marrow, dental pulp, amniotic fluid, umbilical cord, and adipose tissue. The isolation of MSCs from these sources is very straightforward, and MSCs are easily expandable in vitro. In clinical settings, they can be administered locally near the site of the injured tissue or directly into the blood (intravenous/intra-arterial) as systemic administration.
Characterizing MSCs is essential to confirm their identity and quality. The International Society for Cellular Therapy (ISCT) has established minimal criteria to define MSCs, including adherence to plastic, expression of specific surface markers (CD73, CD90, CD105), and absence of hematopoietic markers (CD45, CD34, CD14, or CD11b, CD79a, or CD19, HLA-DR) [13].
MSCs have already received approval from the U.S. Food and Drug Administration (FDA) for some clinical applications. For instance, BM-MSCs are used to treat complications arising from hematopoietic stem cell transplantation, such as graft-versus-host disease (GVHD) [14][36]. BM-MSCs have immunomodulatory properties, suppressing the immune response and promoting tissue repair. Promising results from clinical trials led to FDA approval of MSC-based therapies, and ongoing investigations explore their potential in cartilage and bone repair, wound healing, and autoimmune disorders [9].
2.1. MSCs in Craniofacial and Oral Soft Tissue Regeneration
Craniofacial and oral soft tissue regeneration aims to restore damaged or lost tissues in the head and face region resulting from trauma, congenital conditions, surgical interventions, or cancer resections [15][37]. This anatomically complex area includes craniofacial districts such as the maxillofacial region, oral cavity, mandibular area, and facial bones. Injuries to these tissues can have significant functional and aesthetic consequences, impacting speech, chewing, oral health, and facial aesthetics.
Recent studies have explored the potential of MSCs in craniofacial and oral soft tissue regeneration. For instance, Chen et al. (2017) studied calcium phosphate scaffold and endothelial cell co-culture with MSCs from various sources for their respective angiogenic and osteogenic characteristics in vivo and demonstrated that these constructs are promising for craniofacial bone reconstruction application [16][38]. Srinivasan et al. (2021) demonstrated the in vitro bone regenerative capacity of MSCs from human neural crest stem cells in comparison to bone marrow-derived MSCs for craniofacial bone reconstruction [17][39]. The embryonic origin of MSCs was related to the greater regenerative capacity as it resulted in enhanced mineralization and differential expression of genes linked to FGF in a 3D polycaprolactone-tricalcium phosphate (PCL-TCP) scaffold system.
Researchers have investigated that the combination of MSC-derived exosomes with scaffolds promotes tissue repair and regeneration in craniofacial applications without cell transplantation [18][40]. The study concludes that the exosomes from dental pulp-derived mesenchymal stem cells (DP-MSCs) cause osteogenic differentiation and mineralization of bone marrow stromal cells when released in a controlled manner from a biodegradable scaffold and facilitate craniofacial bone reconstruction. Similarly, Liu et al. (2021) demonstrated, in a cell-free approach, that exosomes generated from MSCs had a superior effect on angiogenesis, effectively promoting craniofacial soft tissue regeneration [19][41]. These data provide a novel strategy to use MSCs in regenerative medicine of oral tissue repair. Hydrogels have also received remarkable attention due to their capacity for stimulating bone reconstruction in craniofacial regeneration [20][42]. Chu et al. (2021) also utilized moldable gelatin-nanohydroxyapatite cryogel with allogeneic BM-MSCs for craniofacial bone regeneration [21][43].
