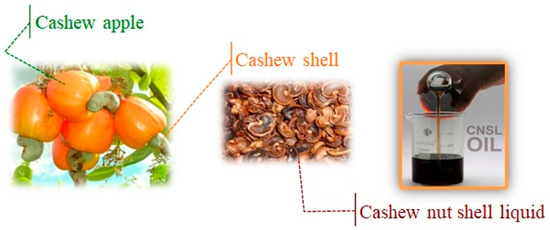
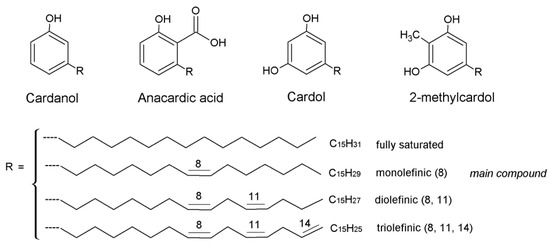
Cashew nut shell liquid (CNSL), obtained as a byproduct of the cashew industry, represents an important natural source of phenolic compounds, with important environmental benefits due to the large availability and low cost of the unique renewable starting material, that can be used as an alternative to synthetic substances in many industrial applications. The peculiarity of the functional groups of CNSL components, such as phenolic hydroxyl, the aromatic ring, acid functionality, and unsaturation(s) in the C15 alkyl side chain, permitted the design of interesting nanostructures. Cardanol (CA), anacardic acid (AA), and cardol (CD), opportunely isolated from CNSL, served as building blocks for generating an amazing class of nanomaterials with chemical, physical, and morphological properties that can be tuned in view of their applications, particularly focused on their bioactive properties.
- CNSL
- cardanol
- anacardic acid
- nanostructures
- green chemistry
- renewable materials
1. Introduction
2. CNSL-Based Nanomaterials
2.1. Cardanol-Based Nanomaterials
Some examples of CA-based metal nanomaterials have been produced via coordination polymerization of the phenolic compound with metal ions or molecules derived from chemical modifications of CA that have shown very versatile properties (optical, photophysical, electrochemical, etc.) and are used to prepare composite nanomaterials with metal oxides. A promising approach, applied to water purification, is heterogeneous photocatalysis using nanostructured semiconductors. In this context, nanocomposite materials based on ZnO nanostructures, impregnated with CA-derived lipophilic porphyrins (H2Pp-metal-free and CuPp-copper porphyrin), provide an alternative technology to efficiently remove toxic substances from water under environmental conditions [6]. In this way, nanomaterials with a diameter of 55 nm were obtained. Moreover, FTIR studies confirm the noncovalent nature of the interactions between CA-porphyrins and ZnO. The photocatalytic activity was investigated via the degradation of rhodamine B (RhB) in an aqueous solution under visible-light irradiation and natural sunlight. Porphyrins are photosensitizing agents for semiconductors; thus, the composite nanomaterials showed better absorption in the visible region than bare ZnO did.
An advanced functional material, like a metal–organic framework (MOF), was produced via the microwave-assisted synthesis of a renewable organic ligand CA and nontoxic endogenous cation Mn(II) bivalent salt [7]. The synthesis was carried out by a “solvent-free in situ” approach, and FTIR spectroscopy was used to confirm the structure and verify the curing of the material (MnIIMicCol). The morphological characterization of the nanomaterial investigated through XRD, optical microscopy, SEM, and TEM showed an amorphous and layered morphology and mesoporous (pore diameter of 8.0286 nm) behavior. The thermal behavior measured by the TGA/DTG/DSC techniques confirmed a high inherent thermal stability. Antibacterial activity was tested against Gram-negative (E. coli and K. pneumoniae) and Gram-positive (B. subtilis and S. aureus) bacterial strains, revealing two bactericidal mechanisms: (i) damage to the bacterial cell membrane and (ii) the production of reactive oxygen species (ROS), which may cause oxidative stress on bacteria cells and damage to both DNA and RNA. The excellent chemical–physical properties and the moderate antibacterial activity make this nanostructured MOF usable in thermally stable (up to 230–250 °C) antimicrobial coating materials.
In newer research, sheet-like nano-biocomposites, based on CA thermosetting resin [8], were obtained. The inclusion of cellulose nanofibrils and nanoplatelets of expanded graphite improved in synergy the flammability, thermal, mechanical, and water absorption properties of nano-biocomposites, required for applicability in coating systems and automotive applications, where weight reduction and a reduction in VOCs in the environment are of great importance. The assembly of this composite material allowed for improving the dispersion of nanofiller cellulose nanofibrils with a high specific surface area and a high percentage of exposed atoms on their surfaces. Furthermore, the CA resin showed a stabilizing effect from the expanded graphite in the nanosheets.
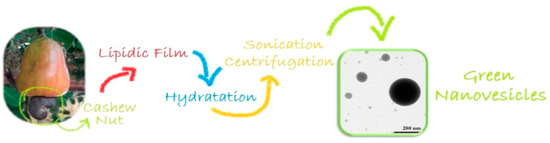
The versatile behavior of CA and its derivative small molecules were found to be attractive in functional soft nanomaterials research to generate self-assembled morphologies down to 100 nm dimensions, such as nanotubes, nanofibers, gels, surfactants, and liquid crystals [9][10]. Gels are systems delicately balanced between molecules’ precipitation and solubilization in a solvent that, self-assembling through noncovalent interactions, form a fibrous network that traps the solvent through capillary forces and resists the flow of the medium. Pyrene-coupled coumarin derivatives with varying hydrophobic chains have been synthesized via aldol condensation, starting from CA-aldehyde, obtained through electrophilic aromatic substitution reactions [11]. The formation of transparent fluorescent organogels occurred via supramolecular self-assembly through the π–π stacking of pyrene units and hydrogen bonding. In particular, absorbance and emission and 1HNMR studies showed that hydrogen bonding between carbonyl groups of coumarin coupled pyrene with the hydroxyl group of a solvent, and π–π stacking interactions have driven the self-aggregation and gel formation processes. The presence of saturated and unsaturated hydrophobic tails affects the gelation efficiency tested in different solvents, strongly influencing the optical properties of π-conjugated derivatives. From these results, self-assembly nanoflakes were derived, and in vitro fluorescence imaging reveals that these compounds inhibit the proliferation of PC3 prostate cancer cells, making them potentially applicable in the cell imaging field. Another study reported the synthesis of coumarin-tris-based amphiphiles, which in turn have been derived from CA [12]. This small amphiphilic system showed the ability to form a stable supramolecular hydrogel sensitive to external stimuli such as pH or the presence of the biologically important Fe3+ ion. Optical microscopy and high-resolution transmission electron microscopy (HRTEM) investigations revealed a reversible morphological transition from self-assembled gels at neutral and basic pH levels to vesicles and nanotubes when pH is acidic. 1HNMR and XRD studies suggested that the π–π stacking interactions and hydrogen bonding were the driving forces for the gelation process.
2.2. Anacardic Acid-Based Nanomaterials
3. Conclusions and Perspectives
The increasing use of agro-industry wastes as raw materials has opened a window of opportunity for the development of alternative products to the oil industry. The development of bio-based materials and technologies based on the use of bio-renewable resources represents a strategic approach to offset the economic impact related to the frequent oscillation in petroleum prices. CNSL represents an example of a naturally occurring oil that includes both phenolic and alkenyl structural properties. These characteristic functional groups can undergo selective or simultaneous chemical modifications by selecting the most suitable chemical approach according to their desirable properties. The present review highlights the potential use of cardanol, anacardic acid, and cardol isolated from a more complex mixture known as CNSL, or natural and technical CNSLs themselves, as prospective “evergreen natural resources” suitable as building blocks to obtain different classes of nanosystems. Recent advances in nanomaterials based on CNSL have been discussed, including their potential applications, among which are biomedical materials and templates for the preparation of nanostructured systems.References
- Lomonaco, D.; Mele, G.; Mazzetto, S.E. Cashew nutshell liquid (CNSL): From an agro-industrial waste to a sustainable alternative to petrochemical resources. In Cashew Nut Shell Liquid; Springer: Cham, Switzerland, 2017; pp. 19–38.
- Mgaya, J.; Shombe, G.B.; Masikane, S.C.; Mlowe, S.; Mubofu, E.B.; Revaprasadu, N. Cashew nut shell: A potential bio-resource for the production of bio-sourced chemicals, materials and fuels. Green Chem. 2019, 21, 1186–1201.
- Mubofu, E.B.; Mgaya, J.E. Chemical Valorization of Cashew Nut Shell Waste. Top. Curr. Chem. 2018, 376, 8.
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162.
- Leite, A.S.; Islam, M.; Júnior, A.L.G.; Sousa, J.M.C.; Alencar, M.O.B.D.; Paz, M.F.C.J.; Rolim, H.; Medeiros, M.G.F.; Melo-Cavalcante, A.A.; Lopes, J. Pharmacological properties of cashew (Anacardium occidentale). Afr. J. Biotechnol. 2016, 35, 1855–1863.
- Ribeiro, V.G.P.; Marcelo, A.M.P.; da Silva, K.T.; da Silva, F.L.F.; Mota, J.P.F.; Nascimento, J.P.C.D.; Sombra, A.S.B.; da Silva Clemente, C.; Mele, G.; Carbone, L.; et al. New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation. Materials 2017, 10, 1114.
- Zafar, F.; Khan, S.; Mondal, A.H.; Sharmin, E.; RizwanulHaq, Q.M.; Nishat, N. Application of FTIR-ATR spectroscopy to confirm the microwave assisted synthesis and curing of Cashew nut shell liquid derived nanostructured materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117732.
- Nwuzor, I.C.; Chukwuneke, J.L.; Ewulonu, C.M.; Okolie, P.C. Fabrication of cardanol thermosetting resin reinforced with cellulose nanofibril/expanded graphite nano-biocomposites. Ind. Crops Prod. 2022, 187, 115392.
- Balachandran, V.S.; Jadhav, S.R.; Vemula, P.K.; John, G. Recent advances in cardanol chemistry in a nutshell: From a nut to nanomaterials. Chem. Soc. Rev. 2013, 42, 427.
- John, G.; Vemula, P.K. Design and development of soft nanomaterials from biobased amphiphiles. Soft Matter 2006, 2, 909–914.
- Lalitha, K.; Nagarajan, S. Strongly fluorescent organogels and self-assembled nanostructures from pyrene coupled coumarin derivatives: Application in cell imaging. J. Mater. Chem. B 2015, 3, 5690.
- Lalitha, K.; Prasad, Y.S.; Maheswari, C.U.; Sridharan, V.; John, G.; Nagarajan, S. Stimuli responsive hydrogels derived from a renewable resource: Synthesis, self-assembly in water and application in drug delivery. J. Mater. Chem. B 2015, 3, 5560.
- Bloise, E.; Carbone, L.; Colafemmina, G.; D’Accolti, L.; Mazzetto, S.E.; Vasapollo, G.; Mele, G. First Example of a Lipophilic Porphyrin-Cardanol Hybrid Embedded in a Cardanol-Based Micellar Nanodispersion. Molecules 2012, 17, 12252–12261.
- Bloise, E.; Becerra-Herrera, M.; Mele, G.; Sayago, A.; Carbone, L.; D’Accolti, L.; Mazzetto, S.E.; Vasapollo, G. Sustainable Preparation of Cardanol-Based Nanocarriers with Embedded Natural Phenolic Compounds. ACS Sustain. Chem. Eng. 2014, 2, 1299–1304.
- Behalo, M.S.; Bloise, E.; Carbone, L.; Del Sole, R.; Lomonaco, D.; Mazzetto, S.E.; Mele, G.; Mergola, L. Cardanol-based green nanovesicles with antioxidant and cytotoxic activities. J. Exp. Nanosci. 2016, 11, 1274–1284.
- Di Bello, M.P.; Bloise, E.; Mazzetto, S.E.; Mele, G. Formulation and Chemical Stability in Aqueous Media of Cannabidiol Embedded in Cardanol-Based Nanovesicles. ACS Sustain. Chem. Eng. 2017, 5, 8870–8875.
- Hamad, F.B.; Mubofu, E.B. Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590.
- Ribeiro, V.G.P.; Barreto, A.C.H.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Sousa, E.M.B.; Fechine, P.B.A. Magnetic nanoparticles coated with anacardic acid derived from cashew nut shell liquid. J. Mater. Sci. 2013, 48, 7875–7882.
- Bezerra, T.T.; de Almeida, M.O.; de Amorim Lima, N.M.; de Castro Rodrigues, N.L.; Ribeiro, V.G.P.; Teixeira, M.J.; Carbone, L.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. In vitro antileishmanial activity of sustainable anacardic acid and cardol based silver nanoparticles on L. braziliensis. Int. J. Pharm. 2022, 619, 121698.
- Mubofu, E.B.; Mlowe, S.; Revaprasadu, N. Cashew nut shells as source of chemicals for preparation of chalcogenide nanoparticles. Nanosyst. Phys. Chem. Math. 2016, 7, 724–727.
- Mlowe, S.S.; Pullabhotla, R.R.; Mubofu, E.E.; Ngassapa, F.F.; Revaprasadu, N.N. Low temperature synthesis of anacardic-acid-capped cadmium chalcogenide nanoparticles. Int. Nano Lett. 2014, 4, 106.
- Chacko, A.S.; Prasad, V.S. Self–assembly of Anacardic Acid Derived Cation–modified Montmorillonite into ’Arthropodal’ Branched Nanofibers. Chem. Sel. 2017, 2, 2288–2292.
- Bloise, E.; Di Bello, M.P.; Carbone, L.; Mazzetto, S.E.; Mele, G. Anacardic Acid: A Promising Building Block for the Sustainable Preparation of Vesicular Nanosystems. Waste Biomass Valor. 2021, 12, 4367–4374.
