Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Sergio Nogales.
Hydrogen as an energy vector is going to play an important role in the global energy mix. On the other hand, wastewater management has become a worldwide concern, as urban settlements have been considerably increasing for decades. Consequently, biodigestion to produce biogas (rich in methane) in water treatment plants could be an interesting starting point to obtain a valuable gas that can be converted into hydrogen through steam reforming.
- methane
- anaerobic biodigestion
- catalysis
- coke deposition
- sewage sludge
- hydrothermal carbonization
- membrane reactor
- pressure swing adsorption
- sulfhydric acid
- hydrogen production
1. Introduction
There is an increasing concern (from local to international, from individual to global society) about environmental problems such as waste management and the sustainable use of resources like water. In that sense, the United Nations has established the so-called Sustainable Development Goals (SDG), where many of these issues have been covered, as in the case of Goal 6, “Clean Water and Sanitation”. Indeed, one of the main goal targets included in this point is the need to improve water quality by reducing pollution, eliminating dumping, minimizing the release of hazardous chemicals and materials, halving the proportion of untreated wastewater, and substantially increasing recycling and safe reuse globally by 2030, expanding international cooperation and support to developing countries in water and sanitation-related activities and programs, like wastewater treatment [1]. On the other hand, there are other interesting goals like Goal 7 (Affordable and Clean Energy), 11 (Sustainable Cities and Communities) and 12 (Responsible Consumption and Production), whose specific goal targets are improving energy efficiency, reducing the adverse per capita environmental impact of cities and waste generation reduction through prevention, recycling, and reuse [1].
With this regard, the role of wastewater, especially in urban areas, seems to be a serious concern from an environmental point of view, as not only is it necessary to purify water before discharging it in rivers, but also the management of wastes, such as sewage sludge, is required.
In order to face this challenge, more and more wastewater treatment plants (WWTP) are implemented every year, as can be seen in Figure 1 in the case of France (selected as an example in Europe). Considering this country, wastewater treatment plants increased by around 15% from 2014 to 2021, which is a significant growth, especially considering that France, Germany, Netherlands, Switzerland, Sweden, Finland or Denmark, among others, exceed 99% of people connected to wastewater treatment plants, pointing out the great effort made by these countries to contribute to the correct management of wastewater. Indeed, according to databases such as the Global HydroWASTE database, there are more than 18,000 wastewater treatment plants in Europe, with 3 million kilometers of sewer network across the European Union [2,3][2][3].
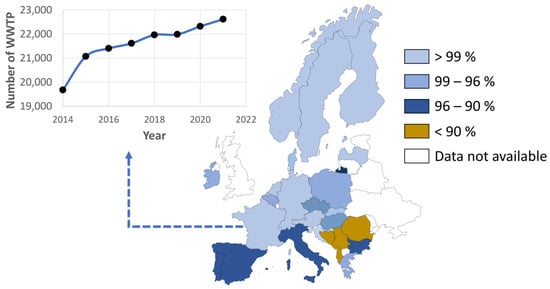
On the other hand, if this increase was observed in such a connected country, it is expected that other countries with a lower percentage of people connected to WWTP (for instance, in Mediterranean countries like Spain and Italy or, especially, in Balkan countries such as Albania, Bosnia and Herzegovina, or Serbia) will increase the implementation of WWTP in the near future, as there is room for improvement to increase connectivity to WWTP by implementing more facilities devoted to this purpose.
Consequently, this increasing trend in the number of wastewater treatment plants in Europe could be equally applied to other developing countries worldwide in the long run [6]. This way, it is estimated that around 110,000 municipal wastewater treatment plants are located in a total of 129 countries, serving up to 2.7 billion people worldwide (approximately 35% of the global population) [7].
This fact points out that there is a global concern about this subject (countries like China or India recently produced up to 40 Mts of sewage per year), with the subsequent increasing interest in using environmental wastes as feedstocks for energy or chemical production [8]. In any case, operating these plants implies the generation of sewage sludge, which is expected to be constantly increased worldwide.
Nevertheless, a wide range of technologies are applied in WWTP, where water treatment is the main objective, but also there are other parallel technologies, such as biodigestion, where biogas (rich in methane) is produced from sewage, which can be used for energy purposes (for instance, electricity conversion) through different devices or technologies (like fuel cells, gas/petrol or diesel engines, gas or steam turbines, etc.) or by different chemical routes, such as methane partial oxidation reforming (POR), dry reforming (DR), autothermal reforming (ATR) or steam reforming (SR) to produce synthesis gas, including hydrogen [9,10,11,12][9][10][11][12].
Specifically, there are more and more WWTPs coupled to biogas production. For instance, in the case of the US, there were 14,780 municipal WWTPs, and 1484 of them digest sludge to produce biogas, according to the US Environmental Protection Agency (USEPA) [13,14][13][14]. This fact points out the room for improvement regarding the implementation of biodigestion in WWTPs around the world, with the foreseeable increase in biogas production in the medium and long term. Consequently, the possibilities for steam reforming of biogas from wastewater are countless, with increasing trends in the use of its raw material.
Hydrogen presents a key role in multisectorial defossilization and decarbonization, which is expected to reach zero net emissions by 2050, with the subsequent increase in hydrogen demand (expected up to USD 12 trillion by 2050) [15,16,17][15][16][17]. This is due to the fact that it implies an excellent environmentally friendly energy carrier, which can be obtained through steam reforming from different natural sources such as biomass [18,19][18][19]. Currently, 48% of total hydrogen production is obtained via natural gas steam reforming, 30% via petroleum fraction, 18% via coal gasification and 4% via electrolysis [20].
This fact proves that the use of biogas could be an interesting alternative for hydrogen production, as it presents some similarities to natural gas (both have methane as their majority compound). In addition, synthesis gas could be suitable for Fischer–Tropsch or methanol synthesis, which are also very interesting chemical routes that could equally enhance the valorization of biogas.
1.1. Wastewater Treatment Plants
Taking into account the context of this cureview articlerent discussion, which is focused on biogas steam reforming, it is vital to understand how a wastewater treatment plant usually works, where there are some stages that are important to understand the relevance of biogas during catalytic steam reforming, as key factors such as quality of biogas (including methane and hydrogen sulfide content) or sewage sludge production will depend on the performance in these facilities. These stages can be categorized into pre-treatment, primary, secondary, and tertiary treatments, which are included in Figure 2.
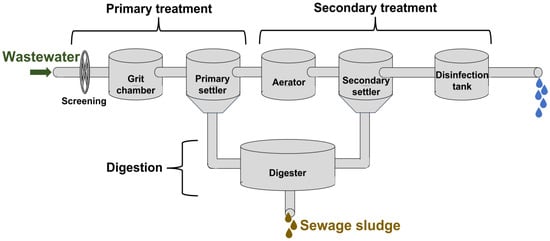
Figure 2.
Main treatments take place in a wastewater treatment plant, including anaerobic digestion, where biogas is produced.
-
Pretreatment: Suspended solids (floating charge, sand, gravel, etc.) that can cause problems in subsequent treatments due to their nature or size are removed during this stage. It includes the separation of large solids, roughing, screening, dilaceration, dewatering, de-oiling, degreasing and pre-aeration, among others.
-
Primary treatment: It implies the separation by physical means of suspended particles not retained in pre-treatment. This treatment can be considered mechanical, mainly based on gravity or mechanical devices to remove pollutants. Consequently, the removal of organic matter can be considered negligible. The main processes corresponding to this stage are sedimentation (primary settling, including coagulation, flocculation, and flotation), gravity separation, and sludge evacuation.
-
Secondary treatment: During this stage, organic matter is removed or at least reduced by using aerobic and anaerobic microorganisms, transforming it into settleable solids that can be easily separated, making it a resourceful technology for sewage sludge management [21]. Specifically, a secondary treatment tank receives the wastewater from the primary treatment after the initial removal of sludge and surface impurities. Before the introduction of wastewater in this secondary treatment tank, 40–60% of solids had already been removed from water, with further removal in this secondary treatment (up to 90%). Thus, there are key steps during secondary treatment, like aeration and sludge sedimentation, included in Table 1.
Tertiary treatment: Finally, this treatment (which is costly) is the most complete procedure for treating wastewater, aiming to remove residual organic load and other pollutants (like P and N) not removed in secondary treatment.
Table 1.
Main steps in secondary treatments.
| Step | Description |
|---|---|
| Aeration | It supplies large amounts of oxygen to wastewater for aerobic bacteria and other micro-organisms, helping to break down many dangerous organic materials in sewage. The resulting clumps, called activated sludge, settle to the bottom of the wastewater. The aerated wastewater is deposited in a secondary sedimentation tank. |
| Secondary sedimentation or clarification | It is usually combined with aeration in a tank: aeration takes place at the top surface, and sludge sedimentation takes place at the bottom. This material is rich in bacteria and other microbes responsible for organic material breakdown and solid, oil or waste removal. |
1.2. Biogas Production in Wastewater Treatment Plants
Biogas is a gaseous fuel with a high percentage of methane (normally above 50%, along with other compounds such as carbon dioxide, nitrogen, or oxygen, among others), which is normally produced through the fermentation of organic matter. In the case of wastewater treatment plants, sewage sludge can be used as an interesting source for biogas production through anaerobic digestion.
Anaerobic digestion is a process used to stabilize sludge and is the natural process of breaking down organic matter by microorganisms in the absence of air [22,23][22][23]. As a consequence, sludge is stabilized, and biogas is generated. Figure 3 shows the main steps that take place during anaerobic digestion [24,25][24][25]:
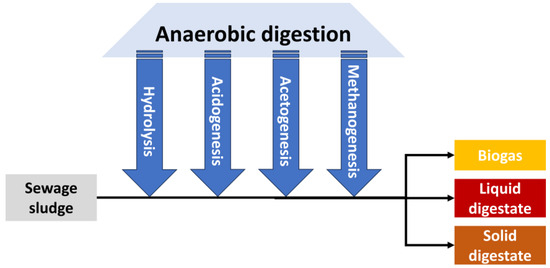
Figure 3.
Main stages during anaerobic digestion of sewage wastewater.
-
Hydrolysis: In this stage, large chains of organic polymers contained in biomass are broken down into smaller constituent parts (monomers such as sugars, amino acids, or fatty acids) and dissolved so that microorganisms in digesters can process them.
-
Acidogenesis: It implies further breakdown of the remaining components by acidogenic bacteria, generating volatile fatty acids, ammonia, carbon dioxide, and hydrogen sulfide (which will play an important role in many aspects of biogas steam reforming), among others.
-
Acetogenesis: The byproducts generated during acidogenesis are further processed by acetogens, mainly producing acetic acid, carbon dioxide and hydrogen (at a lower extent).
-
Methanogenesis: Finally, methanogens convert the previous intermediate products to obtain methane, carbon dioxide and water, which are the majority components of biogas (apart from other traces obtained in previous stages such as H2S, which will play an important and negative role as explained in further sections).
It should be noted that this process is highly dependent on pH, which should be between 6.5 and 8. The remaining material is called digestate, including indigestible material or dead microorganisms, and it can be obtained in liquid and solid states.
In order to select the right design for a digester, frequent organic loading rates and short retention times are essential. Nevertheless, there are plenty of configurations for a digester depending on the requirements of the anaerobic digestion process, the use of additives, the nature of the sewage, etc., obtaining single-phase and multiphase digesters that can use different technologies such as fixed dome method, floating drum method, polythene tube digester plants or earth pit plants.
During anaerobic production to produce biogas, many factors should be considered to obtain high yields with high methane percentages. For instance, temperature could affect microbial communities in biodigesters, clearly affecting biogas production performance [26]. Another aspect to be considered at this point (affecting final quality parameters of biogas such as methane composition) is the possibility of enhancing biogas production through multiple techniques, including organic, inorganic, and biological additives that can enhance microbial activity inside the digester [23,27,28,29,30][23][27][28][29][30].
Depending on the kind of raw material or operational parameters, among other factors such as the kind of digester selected, biogas composition can vary (especially concerning methane percentage, which is indispensable in biogas steam reforming and its yield or industrial design), with the subsequent change in its main properties, as observed in Table 2 [22]. Thus, the nature of the substrate during anaerobic digestion and the design of the biogas production process determines the composition of raw biogas [31]. According to this table, even if a specific biogas source is considered, a wide range of methane composition was found depending on many factors like process conditions or seasonality.
Table 2.
Main characteristics of biogas from different sources.
It should be noted the high and variable concentration of H2S that can be found in anaerobic digestion of SS, up to 2000 ppm, which can play an important role in the final configuration of a biogas steam reforming system, as it will be seen in further sections. Typical natural gas contains (compared to biogas) a higher percentage of methane (89–96%), ethane (1.8–5.1%), lower amounts of carbon dioxide (up to 1%) and oxygen (0.1%), a similar proportion of nitrogen (1.3–5.6%) and a variable composition of hydrogen sulfide (from 0.001 to 0.1%) [10,40][10][40].
1.3. Biogas Steam Reforming
Hydrogen production is a perfect example of green chemistry, contributing to the sustainable growth of population areas [41] with many advantages such as the fact that it is clean energy, it presents a high energy density, its combustion does not generate evolved pollutants, and it is considered one of the most interesting energy types [42]. Pure hydrogen, as well as syngas (a mixture of hydrogen with CO), are gaining importance, as they can be used as an energy carrier or in interesting industrial processes, like methanol synthesis (or more complex compounds) through Fischer–Tropsch reactions.
Apart from primary hydrogen production depending on fossil fuel energy, electrolysis and pyrolysis or more innovative techniques such as bio-hydrogen production [42], one of the possible chemical routes to obtain hydrogen or syngas is steam reforming of methane, present in several industrial gases like natural gas or biogas. Thus, depending on the chemical conditions or the use of additional steps like a membrane reactor, hydrogen at different purity levels can be obtained [10,43][10][43].
Steam reforming of methane or simply steam methane reforming (see Equation (1)) is an endothermic reaction that usually takes place at high temperatures, between 750 and 950 °C and a wide range of pressure values (between 5 and 20 bar).
The presence of CO2 plays an important role in biogas steam reforming, as a second reaction takes place, included in Equation (2).
This way, the simultaneous methane conversion through these chemical routes can lead to biogas bi-reforming or simply biogas steam reforming [44]. It should be noted that another reaction can take place during this process, that is, the water–gas shift reaction (WGS), as observed in Equation (3). Thus, both chemical reactions contribute to a higher yield in hydrogen production and, therefore, higher hydrogen concentrations are found at the reactor outlet.
If Equations (1) and (3) are combined, Equation (4) is obtained:
Consequently, regarding the above, many factors should be considered to optimize biogas steam reforming, such as methane purity, temperature, pressure, the use of catalysts and other purification techniques, such as pressure swing adsorption or membrane reactors.
2. Technology and Chemical Conditions
In this section, general comments about the technology used during biogas steam reforming, as well as the specific circumstances applied to this process compared to other steam reforming processes, are included. This way, there are many studies in the literature with the possible application of mature or emerging technologies to improve biogas steam reforming, mainly focused on biogas quality (for instance, to improve methane percentage through pressure swing adsorption, PSA) or the final product (to improve hydrogen proportion, as in the case of PSA or the use of membrane reactors). Also, other processes such as methanol production or Fischer–Tropsch applied to syngas obtained from biogas steam reforming might be an interesting alternative, especially if H2/CO ratios are suitable for this purpose. In that sense, these processes could avoid costly purification stages as they can use hydrogen and carbon dioxide mixtures directly. The main technologies related to biogas steam reforming are observed in Figure 4. In that sense, there are many treatments that can be carried out to improve the quality of biogas and its products or the performance during steam reforming. For instance, biogas upgrading through different techniques, such as CO2 removal, could be an interesting way to improve biogas steam reforming by increasing CH4 percentage in final biogas [47][45]. In addition, biogas upgrading by biogas recirculation during anaerobic digestion seems to be an effective way to increase methane percentage (up to 90%) and reduce hydrogen sulfide content in final biogas [48][46], which could imply a better performance of this biogas during steam reforming. In any case, upgraded biogas could be equally used as biomethane or an alternative for natural gas if carbon dioxide removal is effective.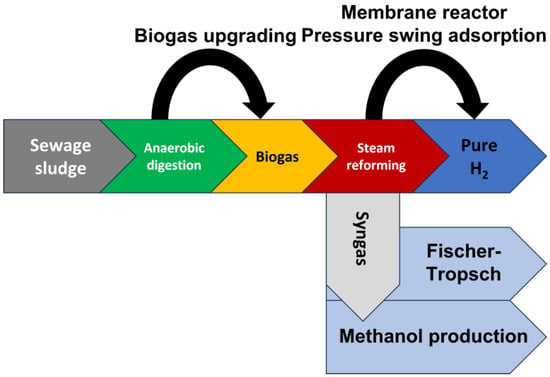
Figure 4.
Main possibilities applied to biogas steam reforming.
2.1. Influence of Chemical Conditions on Biogas Steam Reforming
Regarding the main chemical conditions observed in biogas steam reforming (or biogas bi-reforming), there are some factors that should be considered, such as the effect of temperature, pressure or steam-to-carbon ratio [50][48]. These are the main chemical conditions affecting steam reforming, but there are also other circumstances (as explained throughout this rteviewxt) that could equally affect SR performance, such as the presence of hydrogen sulfide even at low concentrations. Thus, temperature plays an important role, as methane conversion in biogas increases with temperature (from a temperature range of 500–1000 °C), mainly due to the endothermic nature of the main chemical reactions that take place (included in Equations (1) and (2)). In that sense, some studies have proved that biogas steam reforming cannot take place below 350 °C. In addition, in order to avoid coke deposition, which is an undesirable effect that can deactivate catalysts used in this process, high temperatures are recommended. For that purpose, the thermal stability of catalysts should be high, and that is the reason why ceramic-based nickel catalysts are extensively used in this context [12,51][12][49]. Pressure is another interesting aspect, as there seem to be two opposite effects regarding this parameter. In that sense, minimum pressure values (at least 3–5 bar) seem to be required to make the interaction among molecules included in biogas more frequent and effective, whereas excessive pressure (exceeding 20 bar, normally) seems to promote the equilibrium shift towards reagent generation, as SR and WGS reactions show an increase in molecules when products are obtained. Consequently, a pressure range of 3–20 bar is recommended, and a specific choice within this range will depend on other factors such as pressure or S/C ratio. Concerning the steam ratio (another important parameter), it should be noted that the stoichiometric ratio observed in Equations (1) and (3) should be achieved, with higher S/C ratios, ranging from 3 to 6 in most cases observed in the literature, due to the two following reasons:-
The excess of one of the reagents will promote the equilibrium shift towards product generation, increasing methane and carbon monoxide conversion and, therefore, improving hydrogen yield and concentration in the final gas. The higher the purity, the better for further purification steps or treatments.
-
It has been proven that high S/C ratios avoid coke generation (and deposition in some important parts of the reactor, such as catalyst surface or membrane, if they are used for hydrogen purification) during methane (and subsequently biogas) steam reforming. As explained in further sections, coke deposition is one of the most limiting factors in a steam reforming system, as it can contribute to a drastic decrease in the useful life of some components, such as membrane reactors or catalysts.
2.2. Pressure Swing Adsorption
Pressure swing adsorption (PSA) is a technique used to increase the purity of a certain compound included in a mixture of gases. In the case of this revdiscussiew workon, two main purification processes could take place: first, methane upgrade in biogas production in order to improve the performance during biogas steam reforming, and second, hydrogen purification in the mixture of gases obtained after biogas steam reforming. This is a separation process where, at room or ambient temperature, the pressure of different beds (with selective adsorbents, normally microporous or mesoporous solids such as silica gel, zeolite or activated carbon) is increased to trap gas. Thus, gas molecules are linked to the selective adsorbent at high pressure depending on many factors, such as adsorption forces. In the case of hydrogen (see a configuration devoted to hydrogen purification in Figure 5), these forces are weak due to the fact that it is a highly volatile gas with low polarity, whereas other components such as nitrogen, carbon monoxide or carbon dioxide are highly adsorbable in the different beds. Once these gases are adsorbed, pressure in different beds decreases and increases alternatively in order to carry out the desorption of waste gases (by reducing their gas-phase partial pressures within the column so that the adsorbent can be reused) and the subsequent release of high purity hydrogen [47,54][45][52]. In the case of H2 PSA units designed to treat steam reformer synthesis gases (a similar product as expected in biogas steam reforming), each adsorption bed is, in general, configured as a layered bed with the bottom layer near the feed end filled with activated carbon and the top layer near the product end filled with zeolite. The activated carbon layer acts as a protective bed, adsorbing and desorbing mainly CO2 and CH4, whereas the zeolite layer mainly removes CO and N2.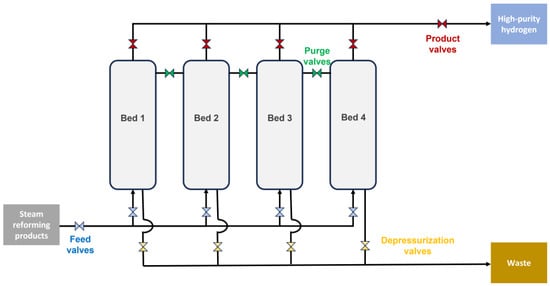
Figure 5.
PSA facilities for biogas steam reforming products.
2.3. Membrane Reactors
The use of membrane reactors (MR) in gas-phase reactions has gained relevance in the last decade. Thus, its application in hydrogen purification processes has equally been interesting, with a tremendous track record found in the literature. Specifically, Pd-based membranes are very interesting in that sense, as they present high selectivity to separate hydrogen from gas streams [57,58][55][56]. Thanks to the higher permeability of hydrogen compared to other compounds included in biogas, such as unreacted methane, carbon dioxide or carbon monoxide, among others, hydrogen generated during steam reforming easily permeates through the membrane, with the subsequent increase in purity, reported to be up to 99% in many cases. This way, due to the removal of one of the products of steam reaction production, the balance of the reactions taking place to produce hydrogen (see Equations (1)–(3)) will be shifted towards product generation, with the subsequent enhancement of the reaction conversion, implying the following advantages:-
Hydrogen is obtained in high purity.
-
The rest of the reagents (in unreacted form), apart from the products, can be easily managed to carry out other chemical routes.
-
The reaction takes place at milder reaction conditions, as lower temperatures or pressure are required, among others.
-
Equally, the amount of active phase in catalysts could be reduced.
-
As a consequence, the energy/economic cost of steam reforming would be drastically reduced, implying a higher competitiveness at the industrial level.
-
Pressure: In this case, there are two opposite effects. On the one hand, according to stoichiometric equations observed in Equations (1) and (2), an increase in mole number towards product generation takes place, making the conversion of reactants unfavorable with pressure. On the other hand, pressure plays a positive effect on the performance of MR, as it favors hydrogen permeation through the membrane. In this case, this latter effect overrides the former effect, obtaining higher conversions with pressure in global terms. However, there is one interesting point to consider, like the pressure resistance of the membrane, which is usually given by the nature of the MR and thickness. Thus, high pressures could promote membrane cracking, generating areas where most gases in the reaction medium, apart from hydrogen, could pass through the MR, which is an undesirable effect as it would imply a considerable decrease in hydrogen purity. To sum up, there are offsetting effects when it comes to pressure, advising high pressures to a certain extent, depending on the kind of membrane.
-
Temperature and space velocity: In this case, higher temperatures favor steam reforming of biogas, as well as a decrease in space velocity increases the residence time of biogas in the reactor, fostering H2 generation and, therefore, increasing its partial pressure, which is suitable for a correct permeation through the membrane. Considering that most membrane reactors are prepared to work at high temperatures and considering that with this configuration, energy savings are assured thanks to lower temperature reactions (between 400–600 °C according to studies included in
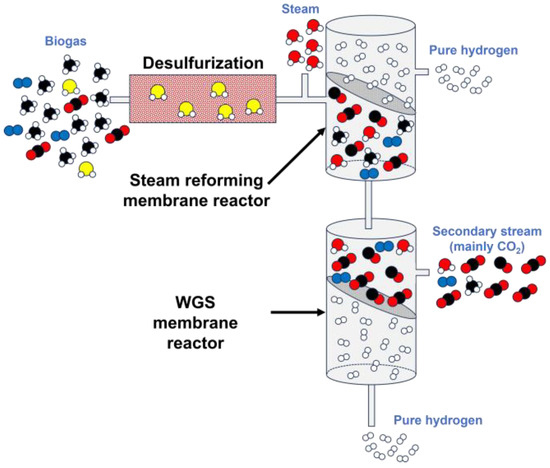
Figure 6.
Typical setting for biogas steam reforming through membrane reactors.
Table 3.
Membrane reactors are used in biogas and SMR according to recent studies.
| Membrane | Chemical Conditions | Comments | Reference |
|---|
- Table 3
- ), there is no concern about the effect of high temperatures on MR integrity. Nevertheless, it would be advisable not to exceed 600 °C to ensure a long membrane lifetime.
| Pd-Au | 420 °C, 300 kPa, 4100 h−1, Ni/Al2O3 catalyst | 30% H2 recovery, 40% CH4 conversion | [60][58] |
| Pd-Ag | 550 °C, 1 bar, water feed of 20% | Up to 73.1 mol of H2 per 100 mol of biogas | [61][59] |
| Pd/Al2O3 | 450 °C, 3.5 bar, S/C = 4, 11,000 h−1, Ni/Al2O3 catalyst | 70% H2 recovery with high purity (>96%) | [62][60] |
| Pd-Au/Al2O3 | 500 °C, 30 atm, 1134 h−1, Ru and Ni/Al2O3 catalyst | 282 mL/min of permeated hydrogen | [63][61] |
| Pd-Au/Al2O3 | 600 °C, 150 kPa, 0.2–1.3 h−1, Rh(1%)/MgAl2 |
- Coke deposition: This is one of the most worrying factors affecting MR performance. Due to the chemical reactions during steam reforming, especially when low S/C ratios are selected, coke deposition on the catalyst or the membrane can take place, hindering a suitable H
- 2 transition through MR.
- H
- 2
- S from biogas: Another problem related to the nature of biogas is the presence, even at low concentrations (up to 100–200 ppm), of hydrogen sulfide. Thus, it is necessary to remove this compound from the original biogas, preferably before steam reforming (see Figure 4 and Figure 6), to avoid problems in biogas steam reforming systems. Obviously, membrane reactors, as a possible component in these kinds of facilities, are no exception, as H2S could provoke poisoning on the surface of the membrane (generating palladium sulfide), possibly leading to the rupture of the membrane layer, especially when they are thin. To avoid this undesirable effect, apart from the obvious removal once biogas is generated, alloys such as Pd-Cu or Pd-Au could be an alternative.
References
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 29 July 2023).
- HydroSHEDS. Hydrowaste Database. Available online: https://www.hydrosheds.org/products/hydrowaste#downloads (accessed on 29 July 2023).
- Waterworld. Europe’s Water/Wastewater in Numbers. Available online: https://waterworld.com/wastewater/article/16201111/analysis-europes-waterwastewater-in-numbers (accessed on 29 July 2023).
- Eurostat. Percentage of People Connected to Wastewater Treatment Plants in Selected Countries in Europe in 2020, by Country. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WW_CON/default/table?lang=en (accessed on 29 July 2023).
- Statista and Ministère de la Transition écologique et de la Cohésion des territoires. Number of Wastewater Treatment Plants in France from 2014 to 2021. Available online: https://www-statista-com.eu1.proxy.openathens.net/statistics/1394264/number-of-wastewater-treatment-plants-france/ (accessed on 29 July 2023).
- Ehalt MacEdo, H.; Lehner, B.; Nicell, J.; Grill, G.; Li, J.; Limtong, A.; Shakya, R. Distribution and Characteristics of Wastewater Treatment Plants within the Global River Network. Earth Syst. Sci. Data 2022, 14, 559–577.
- Adhikari, S.; Halden, R.U. Opportunities and Limits of Wastewater-Based Epidemiology for Tracking Global Health and Attainment of UN Sustainable Development Goals. Environ. Int. 2022, 163, 107217.
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A Critical Review on Biochar for Enhancing Biogas Production from Anaerobic Digestion of Food Waste and Sludge. J. Clean. Prod. 2021, 305, 127143.
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884.
- Prato-Garcia, D.; Robayo-Avendaño, A.; Vasquez-Medrano, R. Hydrogen from Natural Gas and Biogas: Building Bridges for a Sustainable Transition to a Green Economy. Gas Sci. Eng. 2023, 111, 204918.
- Kabeyi, M.J.B.; Olanrewaju, O.A. Technologies for Biogas to Electricity Conversion. Energy Rep. 2022, 8, 774–786.
- Kumar, R.; Kumar, A.; Pal, A. Overview of Hydrogen Production from Biogas Reforming: Technological Advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855.
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Mintz, M.M.; Snyder, S.W. An Overview of Biogas Production and Utilization at Full-Scale Wastewater Treatment Plants (WWTPs) in the United States: Challenges and Opportunities towards Energy-Neutral WWTPs. Renew. Sustain. Energy Rev. 2015, 50, 346–362.
- USEPA. Opportunities for Combined Heat and Power at Wastewater Treatment Facilities: Market Analysis and Lessons from the Field. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.epa.gov/sites/default/files/2015-07/documents/opportunities_for_combined_heat_and_power_at_wastewater_treatment_facilities_market_analysis_and_lessons_from_the_field.pdf (accessed on 14 August 2023).
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen Economy and Sustainable Development Goals: Review and Policy Insights. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506.
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial Decarbonization via Hydrogen: A Critical and Systematic Review of Developments, Socio-Technical Systems and Policy Options. Energy Res. Soc. Sci. 2021, 80, 102208.
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative Assessment of Blue Hydrogen from Steam Methane Reforming, Autothermal Reforming, and Natural Gas Decomposition Technologies for Natural Gas-Producing Regions. Energy Convers. Manag. 2022, 254, 115245.
- Saeidi, S.; Sápi, A.; Khoja, A.H.; Najari, S.; Ayesha, M.; Kónya, Z.; Asare-Bediako, B.B.; Tatarczuk, A.; Hessel, V.; Keil, F.J.; et al. Evolution Paths from Gray to Turquoise Hydrogen via Catalytic Steam Methane Reforming: Current Challenges and Future Developments. Renew. Sustain. Energy Rev. 2023, 183, 113392.
- Sanchez, N.; Rodríguez-Fontalvo, D.; Cifuentes, B.; Cantillo, N.M.; Laverde, M.Á.U.; Cobo, M. Biomass Potential for Producing Power via Green Hydrogen. Energies 2021, 14, 8366.
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen Production via Steam Reforming: A Critical Analysis of MR and RMM Technologies. Membranes 2020, 10, 10.
- Pavičić, J.; Mavar, K.N.; Brkić, V.; Simon, K. Biogas and Biomethane Production and Usage: Technology Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940.
- Bin Khawer, M.U.; Naqvi, S.R.; Ali, I.; Arshad, M.; Juchelková, D.; Anjum, M.W.; Naqvi, M. Anaerobic Digestion of Sewage Sludge for Biogas & Biohydrogen Production: State-of-the-Art Trends and Prospects. Fuel 2022, 329, 125416.
- Deena, S.R.; Vickram, A.S.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K. Enhanced Biogas Production from Food Waste and Activated Sludge Using Advanced Techniques—A Review. Bioresour. Technol. 2022, 355, 127234.
- Nguyen, L.N.; Kumar, J.; Vu, M.T.; Mohammed, J.A.H.; Pathak, N.; Commault, A.S.; Sutherland, D.; Zdarta, J.; Tyagi, V.K.; Nghiem, L.D. Biomethane Production from Anaerobic Co-Digestion at Wastewater Treatment Plants: A Critical Review on Development and Innovations in Biogas Upgrading Techniques. Sci. Total Environ. 2021, 765, 142753.
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555.
- Sudiartha, G.A.W.; Imai, T.; Mamimin, C.; Reungsang, A. Effects of Temperature Shifts on Microbial Communities and Biogas Production: An In-Depth Comparison. Fermentation 2023, 9, 642.
- Vijayakumar, P.; Ayyadurai, S.; Arunachalam, K.D.; Mishra, G.; Chen, W.H.; Juan, J.C.; Naqvi, S.R. Current Technologies of Biochemical Conversion of Food Waste into Biogas Production: A Review. Fuel 2022, 323, 124321.
- Dauknys, R.; Mažeikienė, A. Process Improvement of Biogas Production from Sewage Sludge Applying Iron Oxides-Based Additives. Energies 2023, 16, 3285.
- Liu, M.; Wei, Y.; Leng, X. Improving Biogas Production Using Additives in Anaerobic Digestion: A Review. J. Clean. Prod. 2021, 297, 126666.
- Aromolaran, A.; Sartaj, M.; Abdallah, M. Supplemental Sewage Scum and Organic Municipal Solid Waste Addition to the Anaerobic Digestion of Thickened Waste Activated Sludge: Biomethane Potential and Microbiome Analysis. Fermentation 2023, 9, 237.
- Rafiee, A.; Khalilpour, K.R.; Prest, J.; Skryabin, I. Biogas as an Energy Vector. Biomass Bioenergy 2021, 144, 105935.
- de Nooijer, N.; Gallucci, F.; Pellizzari, E.; Melendez, J.; Pacheco Tanaka, D.A.; Manzolini, G.; van Sint Annaland, M. On Concentration Polarisation in a Fluidized Bed Membrane Reactor for Biogas Steam Reforming: Modelling and Experimental Validation. Chem. Eng. J. 2018, 348, 232–243.
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and Perspectives in Converting Biogas to Transportation Fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152.
- Prask, H.; Fugol, M.; Dyjakon, A.; Głąb, L.; Sowiński, J.; Whitaker, A. The Impact of Sewage Sludge-Sweet Sorghum Blends on the Biogas Production for Energy Purposes. Energies 2023, 16, 2105.
- Vita, A.; Italiano, C.; Fabiano, C.; Laganà, M.; Pino, L. Influence of Ce-Precursor and Fuel on Structure and Catalytic Activity of Combustion Synthesized Ni/CeO2 Catalysts for Biogas Oxidative Steam Reforming. Mater. Chem. Phys. 2015, 163, 337–347.
- del Valle-Zermeño, R.; Romero-Güiza, M.S.; Chimenos, J.M.; Formosa, J.; Mata-Alvarez, J.; Astals, S. Biogas Upgrading Using MSWI Bottom Ash: An Integrated Municipal Solid Waste Management. Renew. Energy 2015, 80, 184–189.
- Zhang, X.; Yang, C.; Zhang, Y.; Xu, Y.; Shang, S.; Yin, Y. Ni–Co Catalyst Derived from Layered Double Hydroxides for Dry Reforming of Methane. Int. J. Hydrogen Energy 2015, 40, 16115–16126.
- Chen, X.; Jiang, J.; Li, K.; Tian, S.; Yan, F. Energy-Efficient Biogas Reforming Process to Produce Syngas: The Enhanced Methane Conversion by O2. Appl. Energy 2017, 185, 687–697.
- Díez-Ramírez, J.; Dorado, F.; Martínez-Valiente, A.; García-Vargas, J.M.; Sánchez, P. Kinetic, Energetic and Exergetic Approach to the Methane Tri-Reforming Process. Int. J. Hydrogen Energy 2016, 41, 19339–19348.
- Viswanathan, B. Energy Sources—Fundamentals of Chemical Conversion Processes and Applications; Elsevier: Amsterdam, The Netherlands, 2016.
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611.
- Wang, H.; Xu, J.; Sheng, L.; Liu, X.; Lu, Y.; Li, W. A Review on Bio-Hydrogen Production Technology. Int. J. Energy Res. 2018, 42, 3442–3453.
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam Reforming of Methane: Current States of Catalyst Design and Process Upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330.
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. IScience Rev. 2020, 23, 101082.
- Upadhyay, A.; Kovalev, A.A.; Zhuravleva, E.A.; Kovalev, D.A.; Litti, Y.V.; Masakapalli, S.K.; Pareek, N.; Vivekanand, V. Recent Development in Physical, Chemical, Biological and Hybrid Biogas Upgradation Techniques. Sustainability 2023, 15, 476.
- Yuan, T.; Zhang, Z.; Lei, Z.; Shimizu, K.; Lee, D.J. A Review on Biogas Upgrading in Anaerobic Digestion Systems Treating Organic Solids and Wastewaters via Biogas Recirculation. Bioresour. Technol. 2022, 344, 126412.
- d’Amore, F.; Pereira, L.M.C.; Campanari, S.; Gazzani, M.; Romano, M.C. A Novel Process for CO2 Capture from Steam Methane Reformer with Molten Carbonate Fuel Cell. Int. J. Hydrogen Energy 2023.
- Brito, J.; Pinto, F.; Ferreira, A.; Soria, M.A.; Madeira, L.M. Steam Reforming of Biomass Gasification Gas for Hydrogen Production: From Thermodynamic Analysis to Experimental Validation. Fuel Process. Technol. 2023, 250, 107859.
- Karpilov, I.; Pashchenko, D. Steam Methane Reforming over a Preheated Packed Bed: Heat and Mass Transfer in a Transient Process. Therm. Sci. Eng. Prog. 2023, 42, 101868.
- García, L. Hydrogen Production by Steam Reforming of Natural Gas and Other Nonrenewable Feedstocks. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 83–107.
- Lee, D.H. Hydrogen Production via the Kværner Process and Plasma Reforming. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 349–391.
- Sircar, S. Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2002, 41, 1389–1392.
- Luberti, M.; Ahn, H. Review of Polybed Pressure Swing Adsorption for Hydrogen Purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933.
- Nikolic, D.; Giovanoglou, A.; Georgiadis, M.C.; Kikkinides, E.S. Hydrogen Purification by Pressure Swing Adsorption. In Proceedings of the 10th conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction (PRES) 2007, Ischia Island, Italy, 24–27 June 2007.
- Peters, T.; Caravella, A. Pd-Based Membranes: Overview and Perspectives. Membranes 2019, 9, 25.
- Arratibel Plazaola, A.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51.
- Amiri, T.Y.; Ghasemzageh, K.; Iulianelli, A. Membrane Reactors for Sustainable Hydrogen Production through Steam Reforming of Hydrocarbons: A Review. Chem. Eng. Process.—Process Intensif. 2020, 157, 108148.
- Iulianelli, A.; Alavi, M.; Bagnato, G.; Liguori, S.; Wilcox, J.; Rahimpour, M.R.; Eslamlouyan, R.; Anzelmo, B.; Basile, A. Supported Pd-Au Membrane Reactor for Hydrogen Production: Membrane Preparation, Characterization and Testing. Molecules 2016, 21, 581.
- Parente, M.; Soria, M.A.; Madeira, L.M. Hydrogen and/or Syngas Production through Combined Dry and Steam Reforming of Biogas in a Membrane Reactor: A Thermodynamic Study. Renew. Energy 2020, 157, 1254–1264.
- Iulianelli, A.; Liguori, S.; Huang, Y.; Basile, A. Model Biogas Steam Reforming in a Thin Pd-Supported Membrane Reactor to Generate Clean Hydrogen for Fuel Cells. J. Power Sources 2015, 273, 25–32.
- Yan, P.; Cheng, Y. Design and Operational Considerations of Packed-Bed Membrane Reactor for Distributed Hydrogen Production by Methane Steam Reforming. Int. J. Hydrogen Energy 2022, 47, 36493–36503.
- Iulianelli, A.; Manisco, M.; Bion, N.; Le Valant, A.; Epron, F.; Colpan, C.O.; Esposito, E.; Jansen, J.C.; Gensini, M.; Caravella, A. Sustainable H2 Generation via Steam Reforming of Biogas in Membrane Reactors: H2S Effects on Membrane Performance and Catalytic Activity. Int. J. Hydrogen Energy 2021, 46, 29183–29197.
- Vásquez Castillo, J.M.; Sato, T.; Itoh, N. Effect of Temperature and Pressure on Hydrogen Production from Steam Reforming of Biogas with Pd-Ag Membrane Reactor. Int. J. Hydrogen Energy 2015, 40, 3582–3591.
- Sheu, W.J.; Hsu, Z.W.; Chen, W.H.; Chen, Y.C. Investigation of Steam Methane Reforming in a Pd–Ru Membrane Reactor with a Counter-Current Configuration. Int. J. Hydrogen Energy 2023.
More
