Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Imran Ashraf.
Despite significant improvement in prognosis, myocardial infarction (MI) remains a major cause of morbidity and mortality around the globe. MI is a life-threatening cardiovascular condition that requires prompt diagnosis and appropriate treatment. The combination of ultra-wideband (UWB) radar and MLmachine learning (ML) approaches has shown significant potential to improve the diagnosis of various medical conditions.
- ultra-wideband radar
- cardiovascular disease
- machine learning
1. Introduction
Despite recent developments in the prognosis of diseases, cardiovascular disease (CVD) is a major cause of morbidity and mortality around the globe [1]. The World Health Organization (WHO) reported approximately 17.9 million victims of CVD [2]. CVD is disproportionately prevalent in low- and middle-income countries, where it accounts for approximately 75% of all fatalities [2]. CVD patients experience heart attacks and strokes as the primary causes of mortality, constituting over 80% of all deaths related to CVD. Various notable risk factors, such as an inadequate dietary pattern, insufficient engagement in physical activities, and the consumption of alcohol and tobacco, can lead to an elevated risk of CVD.
Myocardial infarction (MI) is among five manifestations of coronary heart disease (CHD), which include angina pectoris (both stable and unstable), MI, heart failure, and sudden death [3]. Acute MI has been regarded as the most severe manifestation of CHD, with 2.4 million deaths in the United States (US) and 4 million deaths in Europe and Northern Asia [4,5][4][5]. Similarly, the study [2] reports around 8 million deaths from MI annually. MI is characterized by the sudden cessation of coronary artery function as a result of thrombus blockage at the site of atherosclerotic disease, leading to the destruction of heart muscle tissue [6]. While chest discomfort and shortness of breath are recognized as clinical indicators of MI [7], it is important to note that these symptoms and physical markers lack sufficient sensitivity and specificity in the accurate detection of MI. The condition frequently arises from a decrease in oxygen supply to the cardiac tissues, leading to the formation of blood clots in the coronary arteries [8]. MI has the potential to affect various regions of the heart, including the anterior, inferior, septal, posterior, lateral, inferior–lateral, septal–anterior, and posterior–lateral segments [8,9][8][9]. The obstruction-induced insufficiency of nutrients and oxygen leads to myocardial tissue injury [9].
The monitoring of electrocardiograms (ECGs) and timely detection of abnormalities play a crucial role in reducing mortality rates associated with MI. Single-lead portable ECG monitors are becoming increasingly popular due to their affordable price, effectiveness, and user-friendly interface [7]. ECG data have been utilized for CVD detection in several existing studies [10,11][10][11]. Nevertheless, the interpretation of ECG readings can pose challenges due to the intricate and variable dynamics and morphological characteristics of the ECG signal in individuals with MI. The difficulty arises from factors such as the specific area of the heart affected and the extent of myocardial damage. Such factors may lead to delay in the diagnosis or inaccurate diagnosis. The conventional ECG procedure necessitates the placement of electrodes on the patient’s body, which can potentially lead to skin irritation due to the occasional use of conductive gel [12,13][12][13]. In addition, electrodes may lead to several skin complications, including skin allergies [14]. Extended usage of the device may result in the occurrence of adhesion and a decline in signal quality, thereby requiring the replacement of electrodes [14].
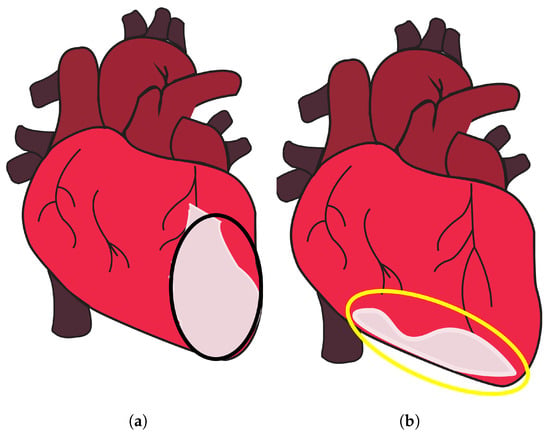
Anterior MI is characterized by myocardial damage that occurs in the front (anterior) region of the heart, specifically affecting the anterior wall of the left ventricle [15], as shown in Figure 1a, encircled in black. The area is supplied with blood by the left anterior descending (LAD) artery, which is a subsidiary of the left coronary artery. The occurrence of an anterior MI can result from the obstruction or occlusion of the LAD [15]. The left ventricle is the largest and strongest chamber of the heart, responsible for pumping oxygenated blood to the body. The contraction of the left ventricle can be significantly impaired as a result of anterior MI [16,17][16][17]. The diminished contractility of the left ventricle may result in a decrease in cardiac output, characterized by a reduction in the heart’s capacity to effectively circulate blood [18]. On the other hand, an inferior MI is characterized by infarction of the myocardium that specifically impacts the lower (inferior) region of the heart [19[19][20],20], as shown in Figure 1b, encircled in yellow. The predominant source of blood supply to this region is the right coronary artery (RCA), with occasional contribution from the left circumflex artery (LCx) [20]. The occurrence of an inferior MI may lead to detrimental effects on both the right ventricle and the inferior region of the left ventricle [19]. The contraction of the right ventricle is accountable for facilitating the transportation of deoxygenated blood to the lungs, where it undergoes oxygenation.
Studies [15,21][15][21] report that patients with anterior MI have a lower ejection fraction than patients with inferior MI. The ejection fraction is a measure of how well the heart pumps blood. A lower ejection fraction means that the heart is not pumping well, which can lead to heart failure [21].
 Recent technological advancements offer promising possibilities for the effective detection of anterior and inferior MI. Ultra-wideband (UWB) is a frequently utilized technology in the monitoring of vital signs [14,23,24,25,26,27][14][23][24][25][26][27]. It has several advantageous features over ECG and other technologies. It does not need direct skin contact for patient monitoring, as ECG does. UWB radar employs low power levels and high data rates to produce high-bandwidth signals characterized by extremely brief pulse durations. The device’s capacity to emit a million nano-pulses per second enables it to effectively identify and monitor min movements and vibrations, including respiration and cardiac activity [26,28,29][26][28][29]. Significantly, the IR-UWB radar system is not dependent on visible light or skin complexion, rendering it suitable for deployment in diverse environmental conditions [28,29][28][29]. Moreover, the emission power of the device is significantly low, with a limited 41.3 dBm/MHz [30,31,32][30][31][32]. This level of emission poses no harm to human health and remains unaffected by Wi-Fi and cell phone transmissions. The IR-UWB radar possesses a notable advantage in comparison to other existing instruments as a result of its non-intrusive characteristics and its capability to effectively penetrate a wide range of materials and barriers [27,28,29,30,31,32,33][27][28][29][30][31][32][33].
Recent technological advancements offer promising possibilities for the effective detection of anterior and inferior MI. Ultra-wideband (UWB) is a frequently utilized technology in the monitoring of vital signs [14,23,24,25,26,27][14][23][24][25][26][27]. It has several advantageous features over ECG and other technologies. It does not need direct skin contact for patient monitoring, as ECG does. UWB radar employs low power levels and high data rates to produce high-bandwidth signals characterized by extremely brief pulse durations. The device’s capacity to emit a million nano-pulses per second enables it to effectively identify and monitor min movements and vibrations, including respiration and cardiac activity [26,28,29][26][28][29]. Significantly, the IR-UWB radar system is not dependent on visible light or skin complexion, rendering it suitable for deployment in diverse environmental conditions [28,29][28][29]. Moreover, the emission power of the device is significantly low, with a limited 41.3 dBm/MHz [30,31,32][30][31][32]. This level of emission poses no harm to human health and remains unaffected by Wi-Fi and cell phone transmissions. The IR-UWB radar possesses a notable advantage in comparison to other existing instruments as a result of its non-intrusive characteristics and its capability to effectively penetrate a wide range of materials and barriers [27,28,29,30,31,32,33][27][28][29][30][31][32][33].

Figure 1. Anterior and inferior MI. (a) Part of the heart where anterior MI occurs, encircled in black, and (b) part of the heart where inferior MI occurs, encircled in yellow, taken from [22].
2. Enhancing Diagnosis by Ultra-Wideband Radar and Machine Learning
MI is a prevalent cause of mortality and morbidity around the globe. The early detection of MI is of utmost importance in the effective management and screening of patients. Unfortunately, the initial diagnosis of patients experiencing chest pain often leads to inappropriate admissions, leading to instances where patients without MI receive treatment, whereas those with MI may be overlooked. The utilization of physical examination, precise ECG findings, evaluation of cardiac troponins, and careful consideration of the patient’s medical history are all crucial factors in the timely identification of MI. AI has revolutionized medical fields by enhancing diagnostic accuracy through image analysis and data-driven disease prediction [34,35,36,37,38][34][35][36][37][38]. Researchers have developed a variety of techniques to detect distinct types of MI. For example, an 11-layer-deep convolutional neural network (CNN) is presented in [39] for automated MI diagnosis. ECG signals dataset from the Physikalisch-Technische Bundesanstalt (PTB) is used for experiments. The Daubechies wavelet 6 mother wavelet function is used to minimize noise and eliminate baseline wander, and the Pan–Tompkins approach is used to find R-peaks. For noisy ECG signals, the accuracy rate, sensitivity, and specificity are 93.53%, 93.71%, and 92.83%, respectively. The average accuracy, sensitivity, and specificity for noise-free ECG signals are 95.22%, 95.49%, and 94.19%, respectively. As proposed in [40], a variety of cardiovascular disorders, including infarction and arrhythmias, can be identified using limited ECG leads. The timely identification of arrhythmias allows healthcare professionals to swiftly respond, potentially mitigating severe outcomes such as strokes or cardiac arrests. The authors use the well-known PTB dataset, which contains 30-s recordings using 12-lead ECG. The ResNet++ model is used with three leads, II, III, and aVF, to identify inferior and anterior wall MI. The proposed model shows F1 scores of 87% and 89%, exceeding ResNet, which has F1 scores of 84% and 87% for inferior and anterior wall MI, respectively. The study [41] developed a strategy for detecting inferior MI quickly and accurately. The method analyzes the segmented multi-lead ECG data with the stationary wavelet transform and splits the signal into separate sub-bands. The multi-lead ECG bands are analyzed for parameters such as estimated sample entropy, normalized sub-band energy, log energy entropy, and median slope. SVM and KNN classifiers are used to detect MI patients. Results indicate that KNN produced a receiver operating characteristic curve (ROC) of 99.45%, sensitivity of 98.67%, specificity of 98.72%, positive predictivity of 98.79%, and accuracy of 98.69%. The results are considerably better when SVM is used, with an ROC of 99.94%, sensitivity of 99.35%, specificity of 98.29%, positive predictivity of 98.41%, and accuracy of 98.84% for the class-oriented approach. The subject-oriented technique, on the other hand, produced an average accuracy of 81.71%, sensitivity of 79.01%, specificity of 79.26%, and positive predictivity of 80.25%. Another study [42] presents an automated technique for detecting Posterior MI (PMI) utilizing a 3-lead vectorcardiogram (VCG). This strategy makes use of electrical conduction properties of heart tissue that vary spatially. A cost-sensitive weighted SVM (WSVM) classifier was devised to solve the issue of class imbalance. The suggested technique was validated using the PTB diagnostic dataset. The WSVM classifier with the radial basis function (RBF) kernel achieved 96.67% test accuracy, 80% sensitivity, and 88.72% geometric mean, respectively. The authors proposed a novel approach in [43] for diagnosis based on the harmonic phase distribution pattern in ECG data. The phase distribution pattern of the Fourier harmonics revealed changes in the shape and timing of the ECG waveform caused by MI. LR and a threshold-based classification approach were used to differentiate between normal and MI participants. The proposed method effectively recognized various types of MI with an average detection accuracy rate of 95.6%, sensitivity of 96.5%, and specificity of 92.7%. The authors described a unique approach for detecting MI from 12 to lead ECGs [44]. This method made use of a one-of-a-kind hybrid network known as the multiple-feature-branch convolutional bidirectional recurrent neural network (MFB-CBRNN). The study also established an optimization approach called lead random mask (LRM) to address potential overfitting difficulties and increase MI detection accuracy. This strategy lowered the likelihood of overfitting and allowed for the use of implicit ensemble techniques such as dropout. The trials were carried out on the PTB dataset, which included 148 people with MI and 52 healthy people, utilizing class- and subject-based fivefold cross-validation. In class-based tests, the MFB-CBRNN attained an outstanding accuracy rate of 99.90% and 93.08% in subject-based trials. Using single-lead ECG data, ref. [45] reported an automatic and exact approach for MI diagnosis and localization. The solution used a sparse autoencoder (SAE)-based feature extraction network to handle the vanishing gradient problem layer by layer. This enabled the network to find optimal feature expressions for the input heartbeats in the absence of labels. The TreeBagger classifier was then used to diagnose MI by merging the outcomes of many decision trees and improving the diagnostic features. On the PTB dataset, this approach surpassed previous algorithms with an accuracy of 99.90%, sensitivity of 99.98%, and specificity of 99.52%. The study [46] used 12-lead ECGs to establish two techniques for MI detection and localization. For feature extraction and classification, the first method used discrete wavelet transform (DWT) in conjunction with principal component analysis (PCA) and a shallow neural network (NN). The second method applied an end-to-end deep learning approach to the processed input signals, employing a CNN. In comparison to prior investigations, the models detected MI with an accuracy of over 98% using smaller feature sets. A multi-channel, multi-scale, two-phase deep learning-based technique for MI detection utilizing VCG signals was proposed in [47]. VCG data was decomposed into five components along each channel, resulting in a multi-channel multi-scale tensor input for a CNN. The technique successfully classified MI cases into distinct sub-diagnoses with 99.58% accuracy, 99.87% specificity, and 99.18% sensitivity, respectively. Similarly, ref. [48] also provided an automatic MI detection system based on a CNN model. The CNN model was optimized using a novel loss function known as the concentrated loss. On the PTB dataset, the suggested technique achieved good accuracy, precision, F1 score, and recall of 98.84%, 98.31%, 97.92%, and 97.63%, respectively. Utilizing ECG data from the PhysioBank database, the study [49] implemented a multi-scale deep learning model with residual networks and attention mechanisms. This model analyzed the 12-lead ECG recordings, calculating and displaying the significance of each lead using the SENet model and Grad-CAM algorithm. By utilizing known MI patterns in specific ECG leads, the model was able to diagnose ten distinct varieties of MI. The outcomes demonstrated extraordinary performance, with average accuracy, sensitivity, and specificity values of 99.98%, 99.94%, and 99.98% for MI detection and 99.79%, 99.88%, and 99.98% for MI localization. The study [50] describes a computerized diagnostic system that detects and classifies five forms of MI from multi-lead ECG signals using a Rough Set classifier. The pathological ECG characteristics associated with MI are extracted, and an information table and knowledgebase are generated. The system identifies critical attributes and generates precise classification rules for MI. It demonstrates robustness via five-fold cross-validation and outperforms existing methods with 99.75% sensitivity and 99.8% accuracy for MI detection and 99.8% accuracy for MI classification. The study [51] examines the significance of accurate ECG in MI diagnosis and ML for automated MI classification. The research utilized Grad-CAM to visualize influential ECG leads and segments in model decisions, with Lead V4 being the most active. Using ECG data from the PTB database, DenseNet and CNN models were developed, achieving high classification accuracy (over 95%). DenseNet outperformed CNN due to its lower computational complexity and higher precision. The study [52] investigates the detection and localization of MI using ECG signals, a non-invasive and cost-effective diagnostic instrument. Using an RF classifier with 100 trees, it obtains remarkable results: an accuracy of 80.98%, a sensitivity of 80.98%, a specificity of 96.32%, a positive predictive value of 79.72%, and an F-score of 79.53% for MI localization in the interpatient scheme, outperforming existing methods. In the interpatient scheme, it achieves a remarkable 96.54% accuracy, 99.74% sensitivity, 96.09% positive predictive value, and an F-score of 97.88% for MI detection. Even with only six chest leads, the method obtains an interpatient detection accuracy of 96.68%. The study presented in [53] aims to increase the efficacy of MI detection by reconfiguring localization as a multilabel classification problem. ST-deviation, T wave amplitude, and R-S ratios are extracted and implemented in an RF chain classifier with five target classes representing MI locations. The method obtains an impressive overall hamming accuracy of 81.49% in cross-validation tests, with the maximum accuracy for the anterior class at 97.72%.References
- Ahsan, M.M.; Mahmud, M.P.; Saha, P.K.; Gupta, K.D.; Siddique, Z. Effect of data scaling methods on machine learning algorithms and model performance. Technologies 2021, 9, 52.
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 August 2023).
- Mendis, S.; Thygesen, K.; Kuulasmaa, K.; Giampaoli, S.; Mähönen, M.; Ngu Blackett, K.; Lisheng, L.; Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction, 2011. World Health Organization definition of myocardial infarction: 2008–09 revision. Int. J. Epidemiol. 2011, 40, 139–146.
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959.
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210.
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528.
- Bęćkowski, M. CARDIOLOGYAcute coronary syndromes in young women—The scale of the problem and the associated risks. Kardiochirurgia Torakochirurgia Pol. J. Thorac. Cardiovasc. Surg. 2015, 12, 134–138.
- Hurst, J.W.; Walsh, R.A.; Fuster, V.; Fang, J.C. Hurst’s the Heart Manual of Cardiology; McGraw-Hill: New York, NY, USA, 2013.
- Yang, H. Multiscale recurrence quantification analysis of spatial cardiac vectorcardiogram signals. IEEE Trans. Biomed. Eng. 2010, 58, 339–347.
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478.
- Hasan, N.I.; Bhattacharjee, A. Deep learning approach to cardiovascular disease classification employing modified ECG signal from empirical mode decomposition. Biomed. Signal Process. Control. 2019, 52, 128–140.
- Nemati, E.; Deen, M.J.; Mondal, T. A wireless wearable ECG sensor for long-term applications. IEEE Commun. Mag. 2012, 50, 36–43.
- Khairuddin, A.; Azir, K.K.; Kan, P.E. Design and development of intelligent electrodes for future digital health monitoring: A review. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 318, p. 012073.
- Crawford, J.; Doherty, L. Practical Aspects of ECG Recording; M&K Update Ltd.: Keswick, UK, 2012.
- Burns, E.; Buttner, R. Anterior Myocardial Infarction. LitFL. 2023. Available online: https://litfl.com/anterior-myocardial-infarction-ecg-library/ (accessed on 20 May 2023).
- Left Ventricle. Available online: https://www.healthline.com/human-body-maps/circulatory-system (accessed on 20 May 2023).
- Heart Anatomy. Available online: https://www.texasheart.org/heart-health/heart-information-center/topics/heart-anatomy/ (accessed on 20 May 2023).
- Dilated Cardiomyopathy. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/dilated-cardiomyopathy (accessed on 20 May 2023).
- Mehta, S.R.; Eikelboom, J.W.; Natarajan, M.K.; Diaz, R.; Yi, C.; Gibbons, R.J.; Yusuf, S. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J. Am. Coll. Cardiol. 2001, 37, 37–43.
- Warner, M.J.; Tivakaran, V.S. Inferior myocardial infarction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Jernberg, T.; Hasvold, P.; Henriksson, M.; Hjelm, H.; Thuresson, M.; Janzon, M. Cardiovascular risk in post-myocardial infarction patients: Nationwide real world data demonstrate the importance of a long-term perspective. Eur. Heart J. 2015, 36, 1163–1170.
- Firuz, C. Myocardial Infarction. Available online: https://www.slideserve.com/chaka/myocardial-infarction (accessed on 7 September 2014).
- Lee, Y.; Park, J.Y.; Choi, Y.W.; Park, H.K.; Cho, S.H.; Cho, S.H.; Lim, Y.H. A novel non-contact heart rate monitor using impulse-radio ultra-wideband (IR-UWB) radar technology. Sci. Rep. 2018, 8, 13053.
- Ren, L.; Koo, Y.S.; Wang, Y.; Fathy, A.E. Noncontact heartbeat detection using UWB impulse Doppler radar. In Proceedings of the 2015 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), San Diego, CA, USA, 25–28 January 2015; pp. 1–3.
- Siddiqui, H.U.R.; Shahzad, H.F.; Saleem, A.A.; Khan Khakwani, A.B.; Rustam, F.; Lee, E.; Ashraf, I.; Dudley, S. Respiration based non-invasive approach for emotion recognition using impulse radio ultra wide band radar and machine learning. Sensors 2021, 21, 8336.
- Siddiqui, H.U.R.; Raza, A.; Saleem, A.A.; Rustam, F.; Díez, I.d.l.T.; Aray, D.G.; Lipari, V.; Ashraf, I.; Dudley, S. An Approach to Detect Chronic Obstructive Pulmonary Disease Using UWB Radar-Based Temporal and Spectral Features. Diagnostics 2023, 13, 1096.
- Rana, S.P.; Dey, M.; Brown, R.; Siddiqui, H.U.; Dudley, S. Remote Vital Sign Recognition through Machine Learning Augmented UWB. In Proceedings of the 12th European Conference on Antennas and Propagation (EuCAP 2018), London, UK, 9–13 April 2018; pp. 1–5.
- Siddiqui, H.U.R.; Saleem, A.A.; Brown, R.; Bademci, B.; Lee, E.; Rustam, F.; Dudley, S. Non-invasive driver drowsiness detection system. Sensors 2021, 21, 4833.
- Siddiqui, H.U.R.; Saleem, A.A.; Bashir, I.; Zafar, K.; Rustam, F.; Diez, I.d.l.T.; Dudley, S.; Ashraf, I. Respiration-based COPD detection using UWB radar incorporation with machine learning. Electronics 2022, 11, 2875.
- Wang, X.; Dinh, A.; Teng, D. Radar sensing using ultra wideband–design and implementation. In Ultra Wideband-Current Status and Future Trends; BoD: Norderstedt, Germany, 2012; pp. 41–64.
- Tsang, T.K.; El-Gamal, M.N. Ultra-wideband (UWB) communications systems: An overview. In Proceedings of the 3rd International IEEE-NEWCAS Conference, Quebec City, QC, Canada, 19–22 June 2005; pp. 381–386.
- Chong, C.C.; Watanabe, F.; Inamura, H. Potential of UWB technology for the next generation wireless communications. In Proceedings of the 2006 IEEE 9th International Symposium on Spread Spectrum Techniques and Applications, Manaus, Brazil, 28–31 August 2006; pp. 422–429.
- Rana, S.P.; Dey, M.; Siddiqui, H.U.; Tiberi, G.; Ghavami, M.; Dudley, S. UWB localization employing supervised learning method. In Proceedings of the 2017 IEEE 17th International Conference on Ubiquitous Wireless Broadband (ICUWB), Salamanca, Spain, 12–15 September 2017; pp. 1–5.
- Zhao, L.; Li, K.; Pu, B.; Chen, J.; Li, S.; Liao, X. An ultrasound standard plane detection model of fetal head based on multi-task learning and hybrid knowledge graph. Future Gener. Comput. Syst. 2022, 135, 234–243.
- Pu, B.; Li, K.; Li, S.; Zhu, N. Automatic fetal ultrasound standard plane recognition based on deep learning and IIoT. IEEE Trans. Ind. Inform. 2021, 17, 7771–7780.
- Alizadehsani, R.; Khosravi, A.; Roshanzamir, M.; Abdar, M.; Sarrafzadegan, N.; Shafie, D.; Khozeimeh, F.; Shoeibi, A.; Nahavandi, S.; Panahiazar, M.; et al. Coronary artery disease detection using artificial intelligence techniques: A survey of trends, geographical differences and diagnostic features 1991–2020. Comput. Biol. Med. 2021, 128, 104095.
- Subasi, A. Use of artificial intelligence in Alzheimer’s disease detection. In Artificial Intelligence in Precision Health; Elsevier: Amsteram, The Netherlands, 2020; pp. 257–278.
- Chang, V.; Bhavani, V.R.; Xu, A.Q.; Hossain, M. An artificial intelligence model for heart disease detection using machine learning algorithms. Healthc. Anal. 2022, 2, 100016.
- Acharya, U.R.; Fujita, H.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M. Application of deep convolutional neural network for automated detection of myocardial infarction using ECG signals. Inf. Sci. 2017, 415, 190–198.
- Rajan, D.; Beymer, D.; Narayan, G. Generalization Studies of Neural Network Models for Cardiac Disease Detection Using Limited Channel ECG. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; pp. 1–4.
- Sharma, L.D.; Sunkaria, R.K. Inferior myocardial infarction detection using stationary wavelet transform and machine learning approach. Signal Image Video Process. 2018, 12, 199–206.
- Prabhakararao, E.; Dandapat, S. A weighted SVM based approach for automatic detection of posterior myocardial infarction using VCG signals. In Proceedings of the IEEE 2019 National Conference on Communications (NCC), Bangalore, India, 20–23 February 2019; pp. 1–6.
- Sadhukhan, D.; Pal, S.; Mitra, M. Automated identification of myocardial infarction using harmonic phase distribution pattern of ECG data. IEEE Trans. Instrum. Meas. 2018, 67, 2303–2313.
- Liu, W.; Wang, F.; Huang, Q.; Chang, S.; Wang, H.; He, J. MFB-CBRNN: A hybrid network for MI detection using 12-lead ECGs. IEEE J. Biomed. Health Inform. 2019, 24, 503–514.
- Zhang, J.; Lin, F.; Xiong, P.; Du, H.; Zhang, H.; Liu, M.; Hou, Z.; Liu, X. Automated detection and localization of myocardial infarction with staked sparse autoencoder and treebagger. IEEE Access 2019, 7, 70634–70642.
- Jafarian, K.; Vahdat, V.; Salehi, S.; Mobin, M. Automating detection and localization of myocardial infarction using shallow and end-to-end deep neural networks. Appl. Soft Comput. 2020, 93, 106383.
- Karhade, J.; Ghosh, S.K.; Gajbhiye, P.; Tripathy, R.K.; Acharya, U.R. Multichannel multiscale two-stage convolutional neural network for the detection and localization of myocardial infarction using vectorcardiogram signal. Appl. Sci. 2021, 11, 7920.
- Hammad, M.; Alkinani, M.H.; Gupta, B.; Abd El-Latif, A.A. Myocardial infarction detection based on deep neural network on imbalanced data. In Multimedia Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–13.
- Cao, Y.; Liu, W.; Zhang, S.; Xu, L.; Zhu, B.; Cui, H.; Geng, N.; Han, H.; Greenwald, S.E. Detection and localization of myocardial infarction based on multi-scale resnet and attention mechanism. Front. Physiol. 2022, 13, 24.
- Halder, B.; Mitra, S.; Mitra, M. Classification of complete myocardial infarction using rule-based rough set method and rough set explorer system. IETE J. Res. 2022, 68, 85–95.
- Jahmunah, V.; Ng, E.Y.K.; Tan, R.S.; Oh, S.L.; Acharya, U.R. Explainable detection of myocardial infarction using deep learning models with Grad-CAM technique on ECG signals. Comput. Biol. Med. 2022, 146, 105550.
- Moghadam, S.R.; Asl, B.M. Automatic diagnosis and localization of myocardial infarction using morphological features of ECG signal. Biomed. Signal Process. Control 2023, 83, 104671.
- Singh, V.M.; Saran, V.; Kadambi, P. Autonomous Myocardial Infarction Detection from Electrocardiogram with a Multi Label Classification Approach. In Proceedings of the Asian Conference on Machine Learning, PMLR, Istanbul, Turkey, 11–14 November 2023; pp. 911–926.
More
