You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Zodwa Dlamini and Version 2 by Rita Xu.
About 15% of all human cancers have a viral etiology. Although progress has been made, understanding the viral oncogenesis and associated molecular mechanisms remain complex. The discovery of cellular miRNAs has led to major breakthroughs. Interestingly, viruses have also been discovered to encode their own miRNAs. These viral, small, non-coding miRNAs are also known as viral-miRNAs (v-miRNAs).
- viral-miRNAs (v-miRNAs)
- human papillomaviruses (HPV)
1. Introduction
Identifying cellular miRNAs has been essential in understanding cancer biology in recent decades. Viral-miRNAs (v-miRNAs) were identified soon after cellular miRNAs. Although cellular and v-miRNAs have comparable biogenesis pathways, the involvement of v-miRNAs in tumorigenesis remains largely unknown. DNA, RNA, and retroviruses can be found to encode v-miRNAs. According to some studies, oncovirus-associated cancers are more prevalent in immunocompromised people [1]. It has also been reported that tumor-suppressor signaling and innate immune signaling share similar effector proteins and/or pathways, such as p21 and p53. v-miRNAs also play an essential role in viral proliferation and persistence. It has been shown that v-miRNAs and cellular miRNAs can influence both host and virus-derived transcripts [2]. This may be possible if both v-miRNA and cellular miRNA share similar seed sequences (miRNA sequence complementary to the mRNA target, 2-8nt from 5′ to 3′ end) and targeted regulation. The relationship between v-miRNA and cellular miRNA orthologues largely remains to be elucidated. It has also been shown that kshv-mir-K12-11 is similar to hsa-miR-155 [3]. The DNA sequences of kshv-mir-K12-10 and hsa-mir-142-3p have a significant degree of sequence similarity [4]. It has also been shown that the seed sequences of both ebv-miR-BART-5 and hsa-miR-18 are similar [4]. Due to the multifaceted nature employed by the v-miRNAs, these molecules may play an important role in precision oncology, particularly in cancers with a viral etiology. Precision oncology can be defined as the molecular profiling of tumors to identify targetable alterations [5]. Cellular miRNAs have also been identified as potent alterations that can be profiled for use in precision oncology [6].
Cellular factors are exclusively involved in v-miRNA biogenesis and the mature v-mRNAs are exported through exosomes [2][7][2,7]. V-miRNAs have immunomodulatory effects, influencing the host’s innate and adaptive immune systems [7]. These v-miRNAs’ immunomodulatory mechanisms confer viruses with the ability to escape the host’s immunosurveillance. Furthermore, v-miRNAs permit viruses to enter their latent phases. These viruses can thus evade detection by immune surveillance systems, increasing the probability of cancer formation [8]. Chronic infection has been shown to upregulate cell adhesion and migration, the cell cycle, immune response and blocking regulation of critical physiological processes [9]. Viruses have also evolved a complicated symbiotic mechanism for accessing and regulating host transcriptional machinery. The roles of Human Papilloma virus (HPV)-, Hepatitis C virus (HCV)-, Epstein Barr virus (EBV)-, Hepatitis B virus (HBV)-, Merkel cell polyomavirus (MCPyV)-, and Kaposi’s sarcoma associated Herpes virus (KSHV)-encoded v-miRNAs in tumorigenesis, v-miRNAs’ role in immune evasion, v-miRNAs as a diagnostic biomarker and as novel anti-cancer therapeutic targets in precision oncology, and, lastly, the challenges and opportunities associated with v-miRNAs in clinical research. Figure 1A shows oncoviruses and their associated cancers, with EBV and HPV having the highest number of different cancers being associated with infection with these viruses. Figure 1B illustrates the relative abundance of v-miRNAs detected in EBV, KSHV, HPV, HBV, MCPyV and HCV to date. EBV has had the highest number of miRNAs identified in its genome. This is followed by KSHV, with nearly two thirds the number of miRNAs as EBV and HPV, with a significantly lower number: nearly a fifth of the miRNAs identified in EBV. The remaining viruses all have very low numbers of isolated v-miRNAs.
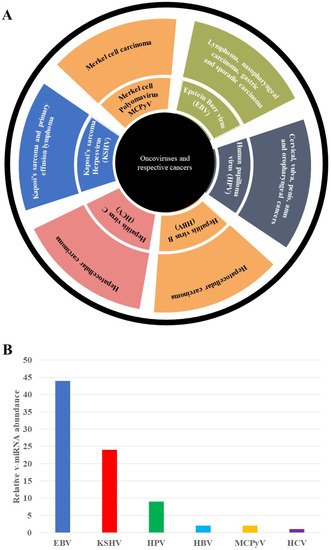
Figure 1. Oncogenic viruses in precision oncology. (A) Summary of oncoviruses and their associated tumors. These oncoviruses include EBV, HPV, KSHV, HBV, HCB and MCPyV. Cancers associated with these viruses include Hodgkin’s lymphoma, cervical cancer, Kaposi sarcoma, liver cancer and Merkel cell carcinomas. (B) Representation of relative v-miRNA abundance. EBV v-miRNAs relative abundance is higher than the other v-miRNAs, followed by KSHV, HPV, HBV, and no HCV* v-miRNA has been confirmed to date [10].
2. Viral-miRNA (v-miRNA) Biogenesis and Role in Tumorigenesis
Viruses utilise the same methods as their host to generate miRNAs that will encode their v-miRNAs. To generate v-miRNAs, Dicer and Drosha must be active [11][12][13][11,12,13]. Primary miRNA is created by the transcribed viral miRNA gene (pri-miRNA). The microprocessor complex DiGeorge syndrome critical region 8 (DGCR8) processes v-pri-miRNA in the nucleus to create pre-v-miRNA with a nucleotide length of 70nt. The Exportin-5 protein transports the pre-v-miRNA from the nucleus to the cytoplasm. In the cytoplasm, Dicer endonuclease transforms pre-v-miRNA into a mature v-miRNA duplex (21–25nt in length). This is subsequently loaded into the RNA-induced silencing complex (RISC), which comprises Argonaute 2 (Ago2) and other components. Figure 2A depicts v-miRNA biogenesis. The v-miRNA-RISC complex then suppresses the target transcript [11][14][11,14]. This repression follows the binding of the v-miRNA to the complementary sequence on the target mRNA. This guides RISC to this target site with the most complementarity, usually in the 3’untranslated region of the miRNA. The seed region is the region that must, in mmost cases, be fully complementary for RISC to act on the mRNA. In a quarter of cases, there may be some incomplete complementarity between the RNA and the seed sequence. Once RISC has been guided to this site, the mRNA may undergo end nucleolytic cleavage through the Ago2 action [15]. V-miRNAs can be found using computational techniques or by sequencing tiny, cloned RNA molecules. The latter strategy has the potential to be more accurate than the former. The sequencing of small, cloned RNA molecules can limit the detection of less abundant v-miRNAs, which can be detected using computational approaches [16][17][16,17]. V-miRNAs have also been reported to regulate the cellular splicing machinery (reviewed in [18]). In addition to this V-miRNAs affect the cellular pathways involved in tumorigenesis. These pathways include cell growth, cell proliferation, cell proliferation and survival, cell death, DNA damage response, and cell cycle arrest. V-miRNAs can also evade the immune system by transitioning from lytic to latent phases, and, due to their micro-size, may escape immune defense (reviewed in [10]). Due to their dual (host and viral) regulatory mechanisms, the network of v-miRNA targets is broad. Figure 2B illustrates v-miRNA-mediated tumorigenic pathways.
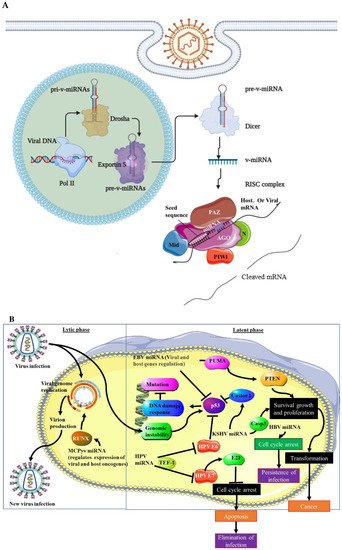
Figure 2. V-miRNAs’ mediated tumourigenesis. (A) Schematic representation of v-miRNA biogenesis. Simliar to cellular miRNAs, v-miRNAs are transcribed as primary v-miRNAs (pri-v-miRNAs) in the nucleus. Pri-v-miRNAs is then cleaved by Drosha into pre-v-miRNA. Exportin 5 exports the pre-v-miRNA to the cytoplasm. Mature v-miRNA is generated from pre-v-miRNA by Dicer cleavage. MiRNA is then loaded into the RISC complex to target mRNA, viral and cellular transcripts [11]. (B) Diagrammatic representation of v-miRNA mediated tumorigenesis. V-miRNAs play an important role in viral latency by targeting key tumour-suppressor genes such as p53 and PTEN, causing genomic instability, and favouring tumorigenic pathways such as cell growth, survival, proliferation and cell transformation, and immune evasion, while inhibiting anti-tumorigenic mechanisms such as cell cycle arrest and apoptosis [10].
