Triple negative breast cancer (TNBC) is a very aggressive subtype of breast cancer that lacks estrogen, progesterone, and HER2 receptor expression. TNBC is thought to be produced by Wnt, Notch, TGF-beta, and VEGF pathway activation, which leads to cell invasion and metastasis. To address this, the use of phytochemicals as a therapeutic option for TNBC has been researched. Plants contain natural compounds known as phytochemicals. Curcumin, resveratrol, and epigallocatechin-3-O-gallate (EGCG) are phytochemicals that have been found to inhibit the pathways that cause TNBC, but their limited bioavailability and lack of clinical evidence for their use as single therapies pose challenges to the use of these phytochemical therapies.
- breast cancer
- triple negative breast cancer
- phytochemicals
1. Introduction
2. Triple Negative Breast Cancer (TNBC) Signaling Pathways
The molecular mechanisms behind TNBC remain unclear despite the fact that several signaling pathways have been identified as playing a role in the development and progression of TNBC. The epidermal growth factor receptor (EGFR) pathway [15][16][37,38], the PI3K/Akt pathway [17][18][19][39,40,41], the RAS/RAF/MEK/ERK network [20][21][42,43], the Wnt/β-catenin pathway [22][23][44,45], and the cyclooxygenase-2 (COX-2) pathway are among the most well-studied signaling pathways associated with TNBC [24][25][46,47]. These signaling pathways implicated in cancer are known to be targeted by various phytochemicals that show evidence of targeting these molecular pathways, including curcumin, resveratrol, green tea polyphenols, sulforaphane, erucin, genistein, genipin, baicalein, quercetin, isoquercitin, vitamin E, parthenolide, dioscin, triptolide, kaempferol, pterostilbene, isoliquiritigenin, and escin [26][48].
2.1. EGFR Pathway
3.1. EGFR Pathway
Tyrosine kinase receptors are growth-factor-binding cell surface receptors. When a growth factor binds to the receptor, the receptor’s kinase domain is activated, resulting in tyrosine phosphorylation and intracellular signaling [27][49]. The EGF receptor tyrosine kinase family is one of the best-studied receptor tyrosine kinase families (also known as the ErbB receptor family). These receptors bind to a variety of growth factors, including EGF. Many signal transduction pathways are activated by ErbB receptors, including the RAS/Erk pathway, the PI3K/AKT pathway, and the JAK/STAT system [28][50]. Additionally, ErbB receptors have the ability to translocate to the nucleus and initiate transcription [29][51]. The EGFR pathway is activated when a ligand binds to the EGFR, causing receptor dimerization and autophosphorylation. This activates downstream signaling pathways that drive cell proliferation, survival, and migration, such as the RAS/RAF/MEK/ERK and PI3K/Akt pathways [30][31][32][52,53,54]. TNBC cells have been found to overexpress EGF, which has been associated with a poor prognosis [15][16][37,38]. The PI3K/Akt signaling pathway is required for cell survival and growth. When this pathway is triggered, Akt is phosphorylated, activating a number of downstream targets involved in cell survival and proliferation [33][34][55,56]. Changes in the PI3K/Akt pathway have been associated with TNBC carcinogenesis and resistance to therapy [17][18][19][39,40,41]. T Molecular docking studies and simulations have been used to study the interaction between EGFR and a multitude of phytochemicals. These include ethanol-soluble phytochemicals from Arnica montana such as caryophyllene oxide and hydroxycadalene c [35][59], furanocoumarin [36][60] and PH-1 (4-methyl-5-oxo-tetrahydrofuran-3-yl acetate), and PH-2 (methyl 4-hydroxy-3-methoxybenzoate) from Polygonum hydropiper [37][61]. The COX-2 pathway is involved in the generation of prostaglandins and has been related to cancer development and progression [38][39][64,65]. COX-2 signaling is initiated by RTK pathways (Figure 1), where it results in the generation of prostaglandins and is responsible for inflammation responses. COX-2 overexpression has been associated with a poor prognosis in numerous malignancies, including TNBC [24][25][46,47]. COX-2 signaling is a component of the EGFR, RTK, and the Wnt signaling pathways (Figure 2).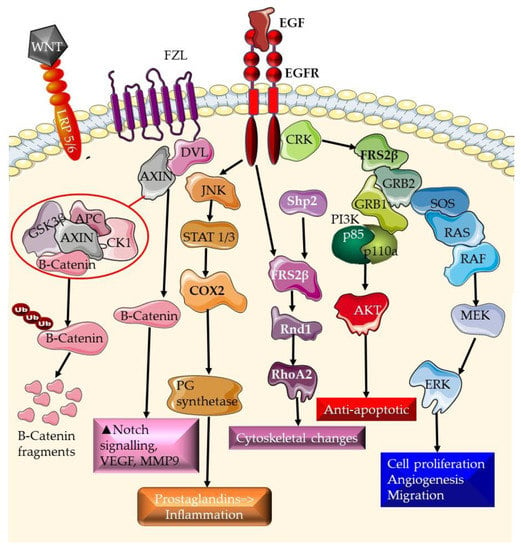
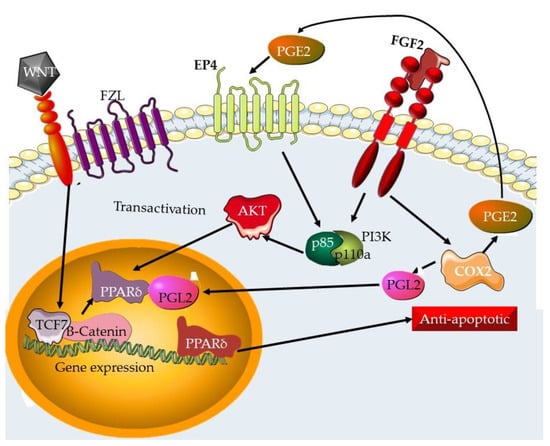
2.2. Wnt/β-Catenin Pathway
3.2. Wnt/β-Catenin Pathway
2.3. Targeting Pathways Involved in Tissue Remodeling, Angiogenesis, and Metastasis in TNBC
3.3. Targeting Pathways Involved in Tissue Remodeling, Angiogenesis, and Metastasis in TNBC
Invasion and metastasis are complicated processes that entail the disruption of normal cellular and tissue connections, followed by cancer cell migration and colonization in distant organs. Several signaling pathways have been linked to the control of invasion and metastasis in TNBC. These include the epithelial-to-mesenchymal transition (EMT) pathway [46][47][48][79,80,81], the integrin pathway [49][50][82,83], the matrix metalloproteinase (MMP) system [51][52][84,85], and the chemokine pathway [53][54][55][86,87,88]. The establishment of new blood vessels via angiogenesis is another important pathway that can be targeted. Tumor tissue, like normal tissue, is unable to develop or spread locally or systemically in the absence of angiogenic support. Blood vessels deliver the oxygen and nutrients needed for growth. Endothelial cells divide and move in response to environmental cues to form blood vessel walls. The activation of the endothelial cell wall of an existing tiny blood vessel (capillary), the production of metalloproteinase enzymes that destroy the proteinaceous extracellular matrix (surrounding tissue), invasion of the matrix, and cell division are the sequential phases of new blood vessel formation. The EMT pathway (Figure 3) is the signaling pathway that controls the process in which epithelial cells lose cell-cell connections and adopt a mesenchymal character, resulting in enhanced motility and invasiveness [56][96]. The EMT pathway has been linked to the development and progression of invasion and metastasis in TNBC, and its activation is linked to a worse prognosis [57][58][97,98].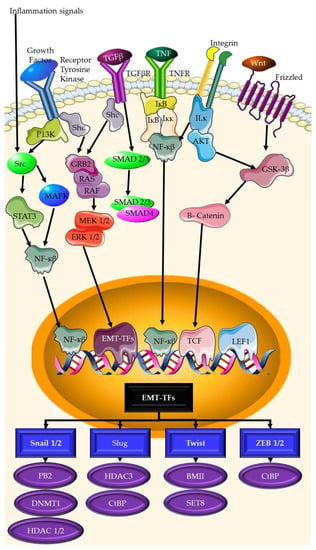
2.4. Phytochemicals Serving as Antioxidants: Cancer Prevention
3.4. Phytochemicals Serving as Antioxidants: Cancer Prevention
Oxidative stress can contribute to the development of cancer through the redox states of many regulatory molecules, signal transduction, regulation of enzyme activity, the control of the cell cycle and proliferation, as well as DNA damage [72][73][112,113]. The activity of many key transcription factors involved in cell cycle regulation can be modulated by ROS. This mainly occurs through oxidative modifications of specific amino acid residues in the DNA-binding motif of the protein or redox-induced changes in phosphorylation status. Depending on the transcription factor in question, redox modifications can serve to either increase or decrease transcriptional activity [74][75][114,115]. Certain oncogenic signals may increase the generation of reactive oxygen species (ROS) due to metabolic activity associated with uncontrolled cell growth and proliferation.2.5. Induction of Apoptosis
3.5. Induction of Apoptosis
One of the most common modes of action for phytochemicals in the treatment of cancer is through the induction or modulation of autophagy and apoptosis. Bcl-2 expression and Bax expression have been shown to be critical in promoting the death of breast cancer cells through the release of cytochrome c and inducing the caspase cascade by the phytochemical Pyranocycloartobiloxanthone A [76][121]. Capsaicin, a significant phytochemical, has been shown to trigger apoptosis in a variety of cancer cells [77][122].3. Current Treatments for Triple Negative Breast Cancer
TNBC is frequently treated surgically, with options including lumpectomy or mastectomy [78][129]. Radiation treatment, in combination with surgery, may be utilized to lower the chance of recurrence in surrounding tissue [79][130]. Chemotherapy is a popular adjuvant treatment that includes taxanes (paclitaxel and docetaxel) [80][131], platinum compound (cisplatin) [81][132], and anthracycline (doxorubicin) [82][133]. These drugs work by targeting and killing rapidly dividing cells, such as cancer cells, but they can also harm healthy cells. These disrupt the cell cycle, causing DNA damage and ultimately cell death, causing nausea, hair loss, and fatigue [83][134]. In recent years, immunotherapy has emerged as a promising therapeutic option for TNBC [84][135]. Immunotherapy, particularly checkpoint inhibitors, work by enhancing the immune system’s capacity to recognize and remove cancer cells. Anti-PD-1 and anti-CTLA-4 antibodies, for example, prevent negative regulatory molecules from acting on the surface of immune cells, allowing the immune system to detect and destroy cancer cells [85][136]. These medications boost the body’s immune system, allowing it to target and eliminate cancer cells [85][86][136,137] Overall, present TNBC treatments have limits and there is a need for the development of new, more effective medicines. The combination of chemotherapy and immunotherapy shows potential for improving TNBC patient outcomes. It is vital to remember that each TNBC patient’s treatment strategy will differ depending on their particular situation and stage of the disease.4. Phytochemicals: An Emerging Therapeutic Option for TNBC
Phytochemicals have recently surfaced as a viable therapy option for TNBC [87][139]. Phytochemicals are plant-derived natural substances that have been demonstrated to have anticancer activities [88][140]. They have gained popularity as potential cancer therapies because of their ability to target many signaling pathways and biological processes that contribute to tumor formation and progression [89][141]. Phytochemicals can fight cancer in a variety of ways, including the induction of apoptosis, the inhibition of angiogenesis, immune system regulation, and cell growth suppression, among others [90][142].
Curcumin, a phytochemical isolated from Curcuma longa, or turmeric, has already been shown to be effective in the treatment of various cancers by inhibiting cell proliferation and inducing apoptosis [91][143]. In the treatment of osteosarcoma, curcumin has been shown to target MAPK/ERK, PI3k/AKT, Wnt/β-catenin, Notch, and microRNA [91][143]. The targeting of the Wnt signaling pathway by curcumin not only inhibits the signaling pathway, but also modifies downstream mediators of the Wnt pathway. These include c-Myc and cyclin D1 [92][144]. Curcumin also has antioxidant, anti-inflammatory, cardio-protective, hepato-protective, and anti-diabetic activities [92][144]. Curcumin interrupts cell proliferation, survival, angiogenesis, and metastasis in TNBC. It is able to act on multiple pathways. These include apoptotic and cell cycle pathways, such as the PI3K/Akt/mTOR pathway, the JAK/STAT pathway, the MAPK pathway, the p53 pathway, and the Wnt/β-catenin pathway [93][145].
The anticancer phytochemical brassinin is derived from cruciferous vegetables and is active against many different types of cancer. When tested against TNBC, this compound reduces the viability of cells and kills endothelial cells before other tumor cells in vitro. Brassinin has negative effects on angiogenesis in TNBC cells, inhibiting proliferation, migration, tube formation, and spheroid sprouting. When it was regularly administered to a dorsal skinfold chamber model of TNBC, it reduced tumor size, microvessel density, and the perfusion of tumor microvessels. The molecular basis of the activities of brassinin include promoting the degradation of Tie2 and fibroblast growth factor receptor 1 [94][149]. Resveratrol, found in red grapes and red wine, is one of the most well-known anticancer compounds [95][96][150,151]. Resveratrol has been found to cause apoptosis, decrease angiogenesis in TNBC cells, and modify the immune system’s response to TNBC tissue and cells [97][98][152,153]. Berberine, is an isoquinoline plant-derived alkaloid, was reported to have anticancer activities, resulting in the creation of synthetic 13-arylalkyl derivatives. Berberine targets the Wnt/β-catenin signaling pathway, inhibiting β-catenin transcriptional activity, and increasing the expression of E-cadherin. Berberine has very low cytotoxicity against normal cells (up to 20 µM). Some of the synthetic compounds based on berberine have higher activity than the natural compound [99][154].