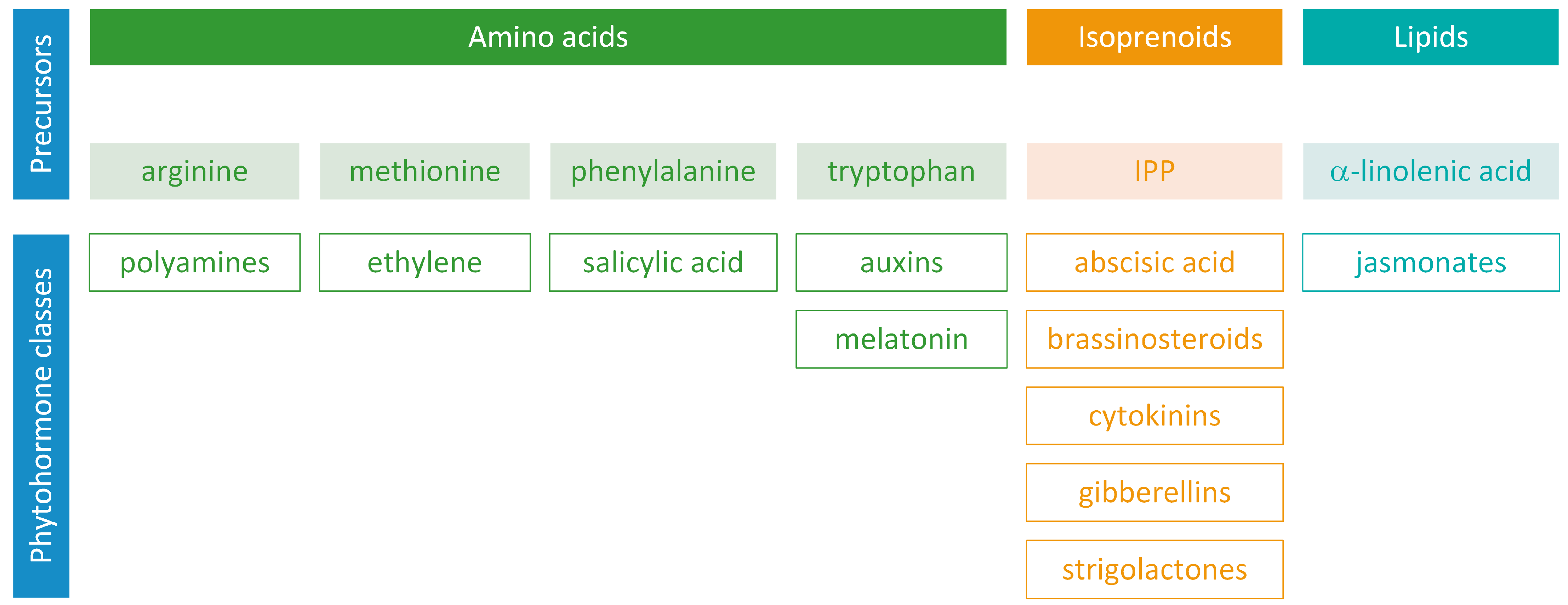
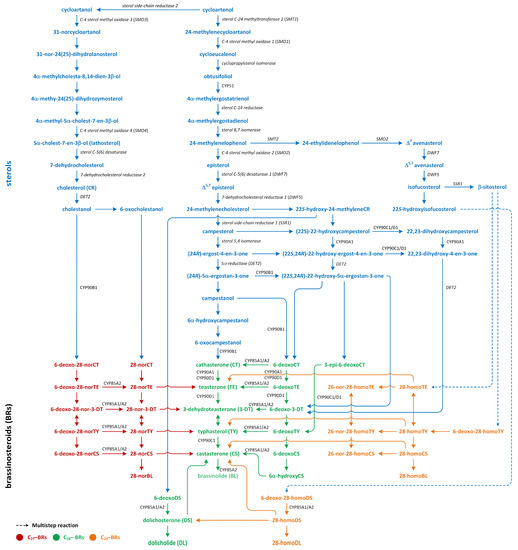
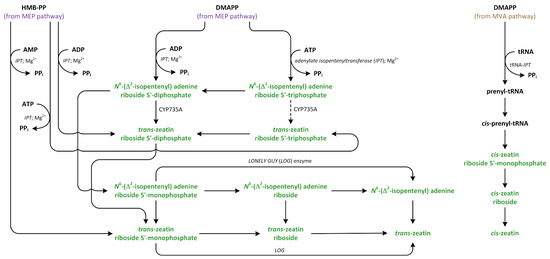
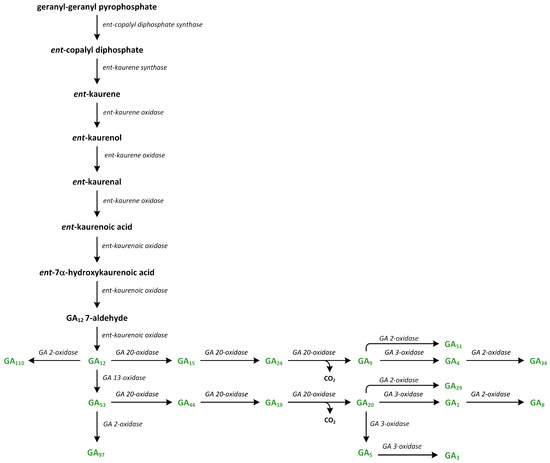
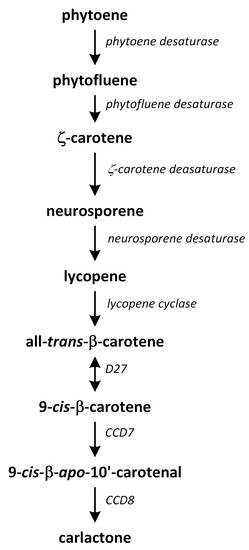
Phytohormones exhibit a wide range of chemical structures, though they primarily originate from three key metabolic precursors: amino acids, isoprenoids, and lipids. Specific amino acids, such as tryptophan, methionine, phenylalanine, and arginine, contribute to the production of various phytohormones, including auxins, melatonin, ethylene, salicylic acid, and polyamines. Isoprenoids are the foundation of five phytohormone categories: cytokinins, brassinosteroids, gibberellins, abscisic acid, and strigolactones. Furthermore, lipids, i.e., α-linolenic acid, function as a precursor for jasmonic acid. The biosynthesis routes of these different plant hormones are intricately complex. Understanding of these processes can greatly enhance our knowledge of how these hormones regulate plant growth, development, and physiology.
1. Introduction
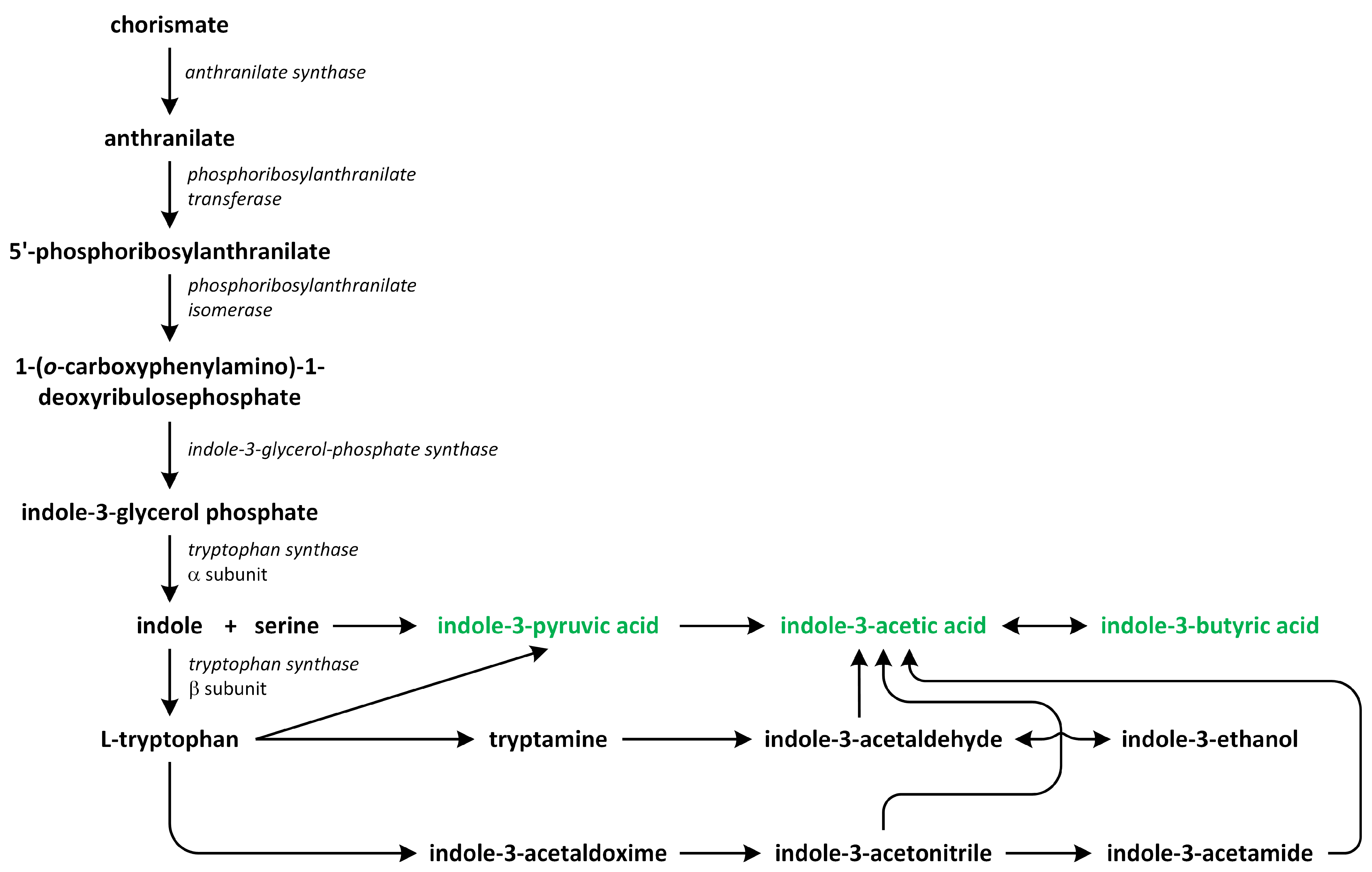
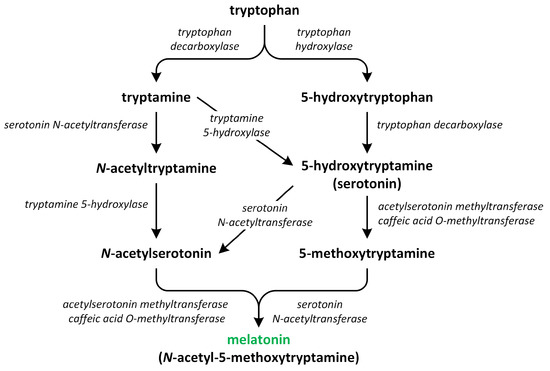
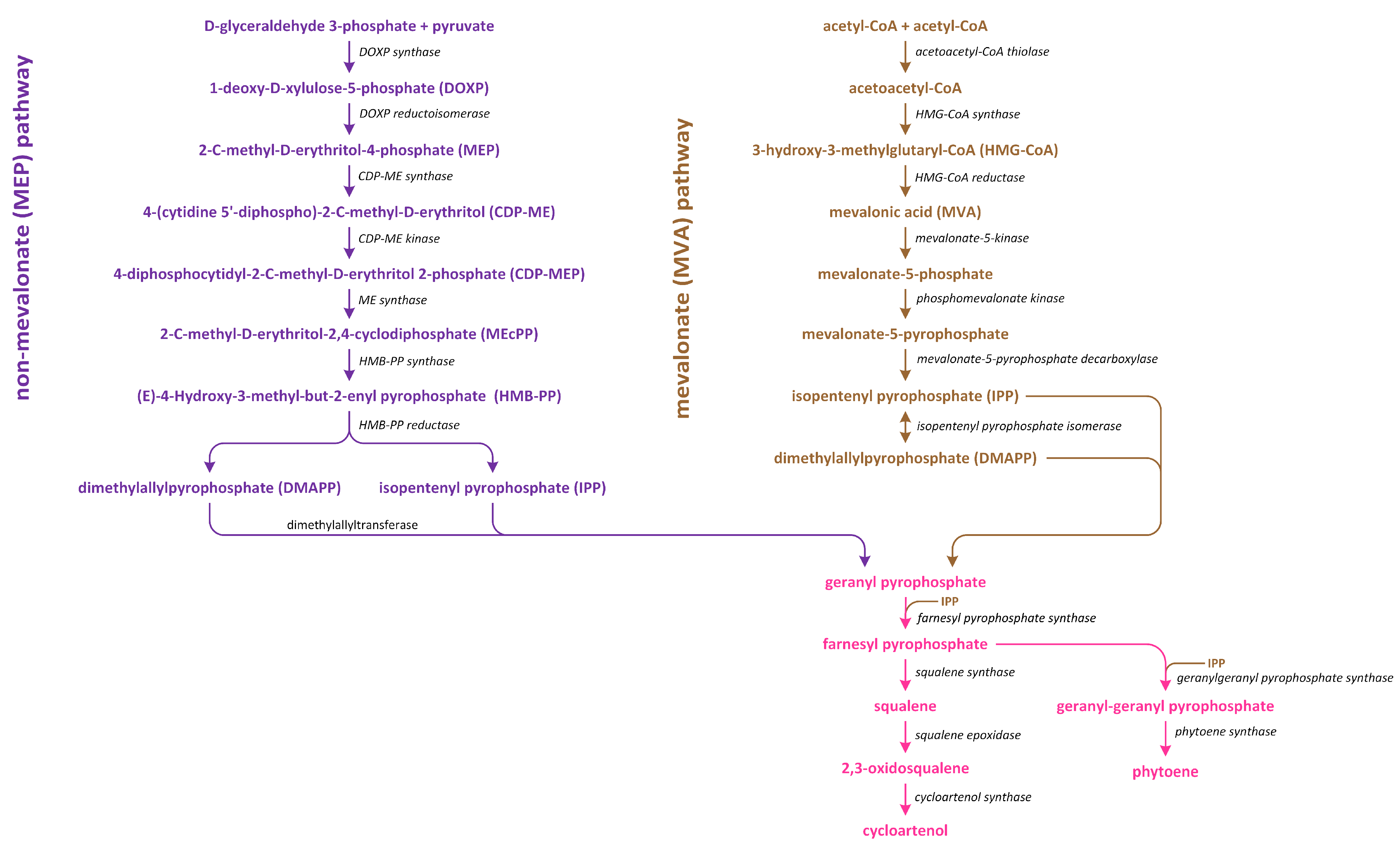
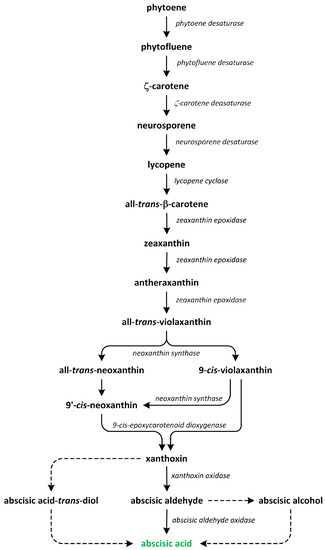
Phytohormones, which are also known as plant hormones, are small, naturally occurring organic compounds that significantly influence the growth, development, defense, productivity, and physiological mechanisms of plants. They also orchestrate various cellular activities within the plant. Even at minimal concentrations, they are operative in plant cells, tissues, and organs. They are found in all vascular plants and a substantial number of non-vascular species (Table 1). From the initial discovery of auxin to the most recent unearthing of strigolactones (SLs), 12 groups of phytohormones—auxins, cytokinins (CKs), gibberellins (GAs), abscisic acid (ABA), ethylene, brassinosteroids (BRs), salicylic acid (SA), jasmonates, polyamines (PAs), melatonin, SLs, and peptide hormones—have been identified in numerous plant species. The various chemical structures of phytohormones are pivotal for their diverse biological functions and biosynthesis (Figure 1):
- auxins and melatonin are indole derivatives;
- ABA is a sesquiterpene;
- ethylene is the simplest alkene;
- CKs are adenine analogues;
- GAs are tetracyclic diterpenoid acids;
- BRs are polyhydroxysteroids;
- jasmonates are derived from fatty acids;
- PAs are aliphatic nitrogenous bases;
- SA is a phenolic organic acid;
- SLs are terpenoid lactones
- [
- 1
- ,
- 2
- ]
- [
- 1][2].
In addition, peptide hormones regulate many developmental and defense processes, such as meristem maintenance, xylem and phloem differentiation, stomata patterning, pollination, embryo and endosperm development, cell division, nodulation, and systematic response
[3]. They are divided into secreted and non-secreted types. Secreted peptide hormones are further divided into post-translationally modified peptides and cysteine-rich peptides. Peptide hormones are synthesized as larger precursor molecules, which are then cleaved to produce active peptides, e.g., CLAVATA3 (CLV3)/Embryo Surrounding Region-Related (CLE), Phytosulfokine (PSK), Plant Peptide-Containing Sulfated Tyrosine (PSY) peptides belong to post-translationally modified peptides; Rapid Alkalinization Factor (RALF) peptides belong to cysteine-rich peptides; Plant Elicitor Peptides (PEP)—to non-secreted peptides. Peptide hormones are also generated by plant pathogens, symbionts, and microbes that interact with plants. They are crucial in establishing a molecular interface that allows them to co-exist with the host plant. These organisms produce effectors that mimic peptide phytohormones and other effectors of pathogens and symbionts. Plant receptors recognize these effectors, which primarily regulate growth rather than defense responses. However, the origin of non-plant peptide phytohormones is still a subject of controversy
[3,4,5,6][3][4][5][6].
As such, plants host diverse phytohormone pathways. Significant advancements have been made in the study of phytohormone biology and synthesis over the past decade. An assortment of new tools and methods has been developed, resulting in the discovery of phytohormone substrates, intermediates, and final products. The biosynthetic pathways of plant hormones have been elucidated, and extensive research has been carried out into the genes found in the plant genome, encoding the enzymes that catalyze the various stages of phytohormone synthesis
[1,2][1][2].
2. Polyamines
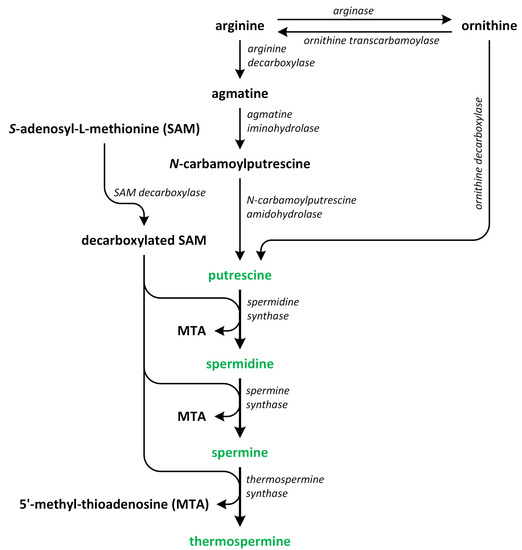
Polyamines (PAs) play a crucial role in plant growth, metabolism, and development. Structurally, these compounds are organic polycationic alkylamines that contain two or more amino groups. PAs such as putrescine (Put), spermidine (Spd), and spermine (Spm) are the most well-studied and recognized PAs found in plants. These three PAs regulate various physiological activities, such as photosynthesis, flower generation, embryogenesis, and organogenesis. They are also accountable for maintaining the stability of nucleic acids, various protein molecules, and the membrane structure. Additionally, they play a substantial role in enhancing the tolerance of many plants to the presence of a variety of abiotic and biotic stress factors, thereby impacting crop yield. Putrescine is a key compound involved in the biosynthesis of PAs. It serves as a common intermediate in the creation of Spd, Spm, and thermospermine. The biosynthesis pathways of Put have been identified in many plants (
Figure 2)
[9,10,11,12][9][10][11][12].