You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Moloud Aflaki Sooreshjani.
Cytokines are secreted proteins that engage the extracellular domains of cell surface receptors and regulate immune response and homeostasis. Cytokines can be classified based on their roles as pro- or anti-inflammatory cytokines or on cellular origin.
- glioma
- cytokines
- tumor microenvironment
1. Introduction
Glioblastoma isocitrate dehydrogenase wild type (GBM IDHwt) is a highly infiltrative malignancy that is poorly controlled by the standard of care that includes surgery, radiotherapy, chemotherapy, and alternating electrical fields [1,2,3,4][1][2][3][4]. Objective response rates (ORR) are very low and are influenced, in large part, by the specific mechanism of action of the therapeutics and their effects on imaging parameters more than on direct tumor cytotoxicity [5]. Current treatment approaches for GBM remain challenging due to tumor heterogeneity [6], an immune-suppressive tumor microenvironment (TME) [7], and the highly infiltrative nature of these tumors [8]. Cytokines are soluble small molecules that mediate the interactions between immune and non-immune cells in the TME and either support pro- or anti-inflammatory responses [9]. Targeted delivery of immune modulatory cytokines through either gene- or cell-based strategies [10,11,12,13][10][11][12][13] may limit adverse effects related to the systemic administration and enhance the efficacy of the treatment [13].
2. Modulation of Tumor Immunogenicity
GBM is a heterogeneous disease that develops a complex TME composed of infiltrating immune cells, vasculature, and fibroblasts exposed to various soluble factors affecting tumor growth [28][14]. These various factors within the TME determine phenotypic features and treatment outcomes. Cancer cells create an immunosuppressive microenvironment through a variety of mechanisms including inducing immune-suppressive macrophages/microglia [29][15] and downregulation of antigen presentation [30][16]. The presence of myeloid-derived suppressor cells (MDSCs) is one of the mechanisms that promote immunosuppressive TME and likely inhibits effective immunotherapy [31][17]. MDSCs migrate as immature cells from the bone marrow to tumors, where they differentiate into mature macrophages and dendritic cells [32,33][18][19]. MDSCs inhibit activation and proliferation of cytotoxic T cells [34][20] through increased expression of arginase-1 [35][21], resulting in increased secretion of IL-10 [36][22] and TGF-β [37][23]. Tumor-associated microglia/macrophages (TAM) impose additional constraints on anti-tumor immunity [38][24] by secreting low levels of pro-inflammatory cytokines [39][25] and compromising T cell function as summarized in Figure 1 and Figure 2 [40][26]. This immune suppression is further compounded by a paucity of T cells within the TME through sequestration in the bone marrow [41][27] and irreversible T cell exhaustion [42][28].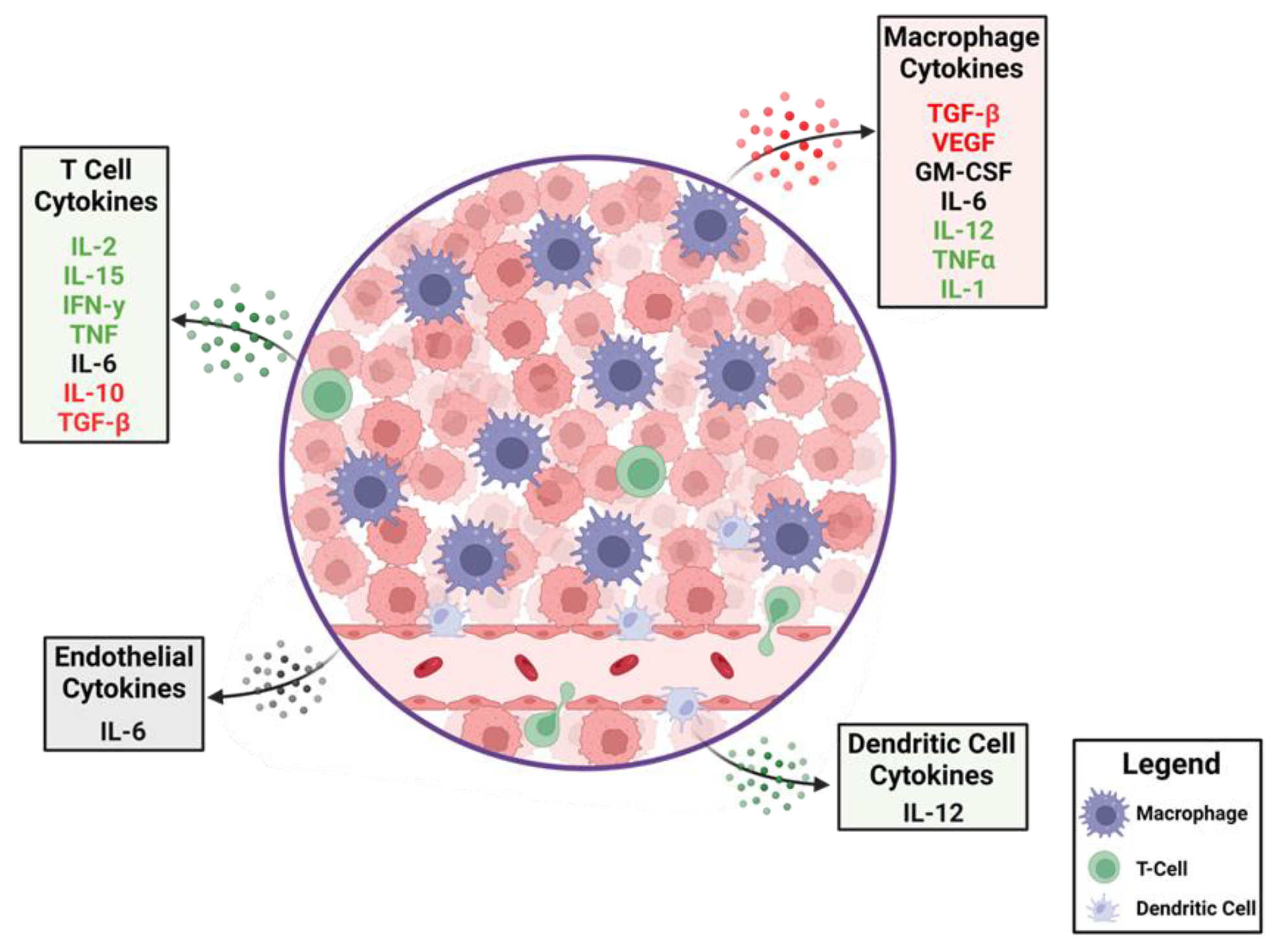
Figure 1. The dynamics of cytokines in the glioblastoma tumor microenvironment (TME). A cartoon depiction of the cytokines that modulate anti-tumor immune responses. Production of immune-suppressive cytokines shown in red are counterbalanced by pro-inflammatory cytokines shown in green. Cytokines that have different immunological roles depending on context are shown in black. A variety of cells within the TME elaborate these cytokines with some, such as macrophages, being abundant, whereas T cells are relatively rare.
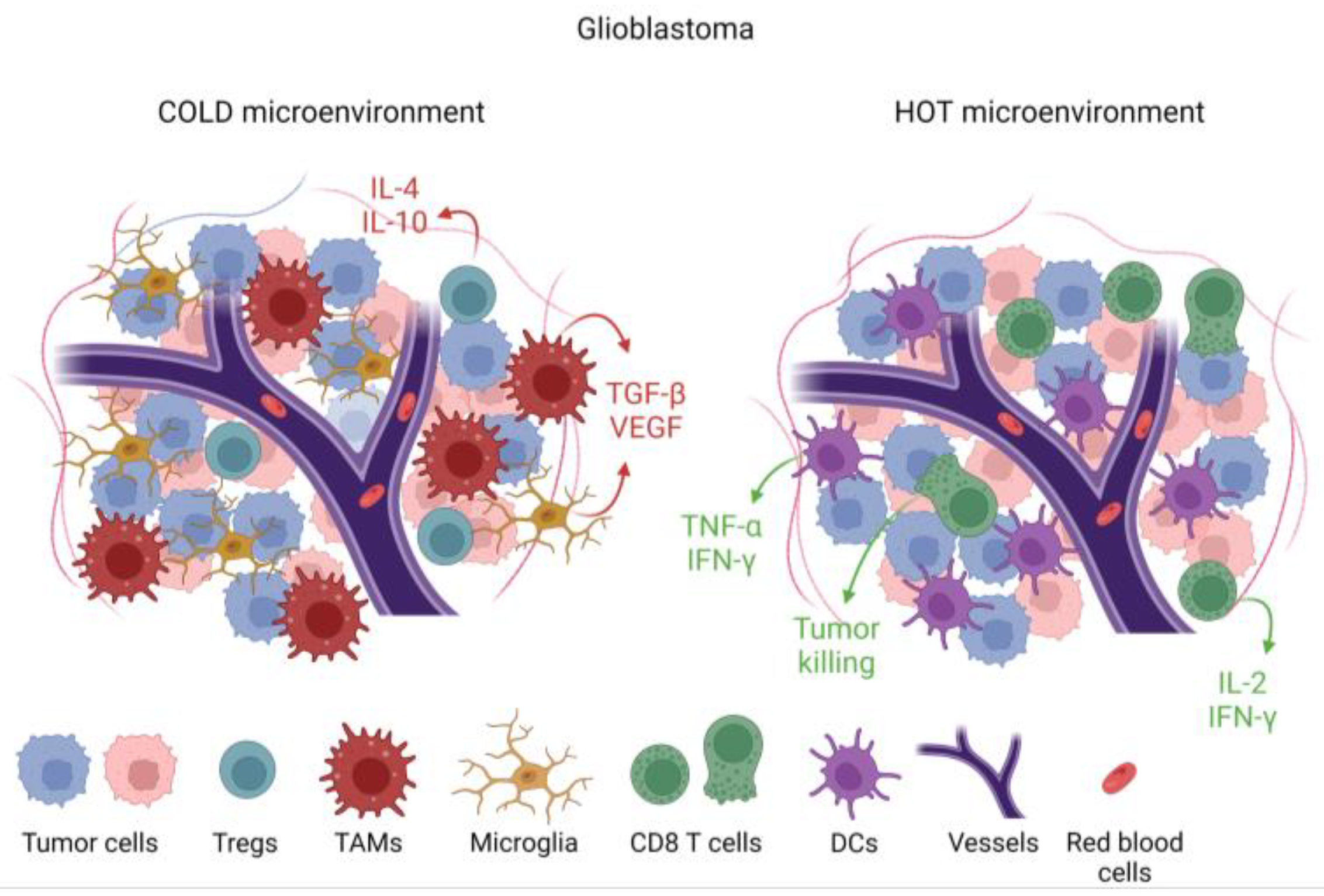
Figure 2. Immunological features of tumors based on cellular and cytokine composition within the tumor microenvironment (TME). Tumors that are devoid of cytotoxic T cells and pro-inflammatory cytokines such as IL-2, IFN-γ, and TNF-α, but with immune-suppressive cytokines such as TGF-β and immune-suppressive cells such as tumor-associated macrophages (TAMs), are designated as immunologically cold. This cold TME is associated with microglia infiltration. In a hot TME, which is rare in glioblastoma, there would be abundant CD8 cytotoxic T cells and dendritic cells alongside pro-inflammatory cytokines.
3. Cytokine Biology
Cytokines are secreted proteins that engage the extracellular domains of cell surface receptors and regulate immune response and homeostasis [9]. Cytokines can be classified based on their roles as pro- or anti-inflammatory cytokines [43][29] or on cellular origin (Table 1). Type 1 (cellular response) cytokines are secreted by CD4+ Th1 and type 2 (humoral response) cytokines are produced by CD4+ Th2 cells [44][30]. Although the immune regulatory effects of cytokines make them compelling candidates for cancer immunotherapy, undesirable side effects and short serum half-life can restrict clinical implementation [45][31]. Cytokine pleiotropy, which refers to the ability of cytokines to act on different cell types in the immune system and peripheral tissues, is also a challenge for clinical translation because of off-target effects [46][32]. Multiple immunomodulatory cytokines have or are being investigated for clinical use, including TGF-β, CSF-1, IL-2, IL-7, IL-10, IL-12, IL-18, IL-21, IL-22, and IFN-α, some of which include glioma patients (Table 2). Only IFN-α and IL-2 have received U.S. Food and Drug Administration (FDA) approval for cancer treatment [47][33]. There has been limited experience with high-dose IL-2 in GBM patients after one subject had a fatal outcome secondary to herniation associated with marked T cell tumor infiltration that has not been reported. Human interferon alpha 2b (IFN-α2b) was approved for the treatment of hairy cell leukemia in 1986 and recombinant IL-2 for treating melanoma and renal cancers in 1992 [47][33]. With these treatments, severe side effects can include capillary leak syndrome and cytokine release syndrome, leading to death in some patients. In many instances, the concentration of the cytokine leads to different effects including unwanted off-target toxicities. As opposed to conventional chemotherapy in which the highest tolerated doses are typically used, efforts need to be directed at the identification of the appropriate dose for the desired physiological result in the case of cytokines. As such, the management of cytokines, including toxicities, is a more subtle process with titration of the dose in contrast to more standard pharmacologic management of an “on/off switch” approach. As such, the management of cytokines is a different concept when juxtaposed with cytotoxic chemotherapy where the intention is to maximize cytotoxicity. Given the toxicity of cytokine-based therapies, considerable effort has been focused on targeting cytokines through cytokine-producing viral vector gene therapy and adoptive transfer of cytokine-producing cells.Table 1.
Cytokine control of the immune system.
| Mediator | Cellular Source | Function |
|---|---|---|
| IL-1 |
Table 2.
Chemokine clinical trials in glioma patients.
.
| Mediator | Phase | Therapeutic Benefit | Side Effects | Reference | |||
|---|---|---|---|---|---|---|---|
| Macrophages, epithelial cells | Pro-inflammatory, macrophage, and Th17 cell activation | ||||||
| IFN-α | 3 | Increase in overall survival in combination with the current standard of care | Seizures and flu-like symptoms | [34] | IL-2 | T cells | Effector T cell and regulatory T cell growth factor |
| 3 | No benefit in combination with radiation and carmustine | Fevers, chills, myalgia, somnolence, confusion, and neurological deficits | [35][36][37] | IL-4 | Th-cells | T and B cell proliferation and B cell differentiation | |
| IFN- α-2a | 2 | No benefit | Dermatological effects | [38] | IL-6 | Macrophages, T cells, endothelial cells | Both pro-inflammatory and immune suppressive, increased antibody production |
| IFN-α-2b (PEG-Intron) | 2 | No benefit in DIPG patients | Well tolerated | [39] | IL-8 | Macrophages, epithelial cells | |
| IFN-β | Recruitment of neutrophils | ||||||
| 2 | No benefit in combination with the current standard of care | Increased neutropenia | [40][41][42][43][44][45 | Th9 cells | Activation of mast cells | ||
| ] | IL-9 | ||||||
| IFN-γ | 2 | No benefit | Well tolerated | [46] | IL-10 | Regulatory T cells, Th9 cells | Immune suppressive, inhibition of Th1 cells |
| IL-12 | 1 | Safety | Well tolerated | [47][48][49] | IL-11 | Fibroblasts, neurons | Immune suppression |
| CXCR4 inhibitor | 1 | Safety | Well tolerated | [50] | IL-12 | Dendritic cells, macrophages | Activation of Th1, induction of interferon from cytotoxic T cells and NK cells |
| CSF-1 inhibitor | 2 | No benefit | Well tolerated | [51] | IL-15 | CD8 T cells, NK cells | Expansion of memory CD8 and NK cells |
| TGF-βR1 | 2 | IL-17 | Th17 cells, NK cells | Promotes neutrophilic inflammation | |||
| Safety | IL-18 | Monocytes, macrophages, dendritic cells | Pro-inflammatory, activation of the Th1 pathway | ||||
| Preserved T cell counts | [ | 52 | ][53] | ||||
| TGF- βR2 | 2 | No benefit | Seizures, edema | [54] | |||
| TNF-α | 1 | Safety | Well tolerated | [55][56] | IL-33 | Macrophages, dendritic cells, mast cells, epithelial cells | Pro-inflammatory, amplification of Th1 and Th2 cells, activation of NK cells |
| GM-CSF | 3 | No benefit | Well tolerated | [1][57][58] | IFN-γ | Th1 cells, cytotoxic T and NK cells | Pro-inflammatory and activates macrophages |
| IL-2 | 1 | No benefit | Fatigue, edema | [59][60][61][ | Tumor necrosis factor | Macrophages, T cells, NK cells | Pro-inflammatory increases vascular permeability |
| GM-CSF | Macrophages, T cells, NK cells, and endothelial cells | Pro-inflammatory but glioma propagating | |||||
| VEGF | Macrophages | Angiogenesis | |||||
| TGF-β | Macrophages, T cells | Immune suppressive | |||||
| CXCL9 | Monocytes, endothelial cells | Recruitment of Th1, NK, and dendritic cells | |||||
| CXCL10 | Monocytes, endothelial cells | Recruitment of macrophages, Th1, and NK cells | |||||
| CXCL12 | Mesenchymal stem cells | Chemotactic for T cells | |||||
| CCL2 | Macrophages, dendritic cells | Recruitment of Th2, monocytes, and dendritic cells | |||||
| 62 | ] | CCL3 | Monocytes, neutrophils, dendritic cells | Recruitment of macrophages, Th2, NK, and dendritic cells | |||
| CCL4 | Macrophages, neutrophils, endothelium | Recruitment of macrophages, Th1 cells, NK, and dendritic cells | |||||
| CXCL13 | B cells | Recruitment of B cells, CD4 T, and dendritic cells |
| Mediator | Phase | Therapeutic Benefit | Side Effects | Reference |
|---|---|---|---|---|
| IFN-α | 3 | Increase in overall survival in combination with the current standard of care | Seizures and flu-like symptoms | [48] |
| 3 | No benefit in combination with radiation and carmustine | Fevers, chills, myalgia, somnolence, confusion, and neurological deficits | [49,50,51] | |
| IFN- α-2a | 2 | No benefit | Dermatological effects | [52] |
| IFN-α-2b (PEG-Intron) | 2 | No benefit in DIPG patients | Well tolerated | [53] |
| IFN-β | 2 | No benefit in combination with the current standard of care | Increased neutropenia | [54,55,56,57,58,59] |
| IFN-γ | 2 | No benefit | Well tolerated | [60] |
| IL-12 | 1 | Safety | Well tolerated | [61,62,63] |
| CXCR4 inhibitor | 1 | Safety | Well tolerated | [64] |
| CSF-1 inhibitor | 2 | No benefit | Well tolerated | [65] |
| TGF-βR1 | 2 | Safety | Preserved T cell counts | [66,67] |
| TGF- βR2 | 2 | No benefit | Seizures, edema | [68] |
| TNF-α | 1 | Safety | Well tolerated | [69,70] |
| GM-CSF | 3 | No benefit | Well tolerated | [1,71,72] |
| IL-2 | 1 | No benefit | Fatigue, edema | [73,74,75,76] |
4. Targeting Pro-Tumoral Cytokines
4.1. Targeting Transforming Growth Factor β (TGF-β)
TGF-β is a cytokine with pleiotropic effects which may play an important role in anti-tumor immune responses [77][63]. TGF-β supports stem-like self-renewal and suppression of immune response [78][64]. TGF-β expression, presumably in the context of the above-described effects, is associated with glioma development and progression [79][65]. In turn, targeting this cytokine is a rational therapeutic approach. A non-randomized phase 1/2 clinical trial (NCT01220271) showed the safety and tolerability of LY2157299, a small molecule inhibitor of TGF-β receptor type I, in combination with temozolomide and radiation in newly diagnosed high-grade gliomas [80][66]. However, treatment of patients with LY2157299 and lomustine did not improve the overall survival (OS) relative to monotherapeutic lomustine in patients with recurrent GBM [81][67]. Another approach for targeting TGF- β involves the use of bintrafusp alfa, a bifunctional protein consisting of an antibody blocking PD-L1 and TGF-β trap [82][68]. Because PD-L1 can be expressed on some types of cancer cells which prevents T cells from killing, targeting two distinct mechanisms of tumor-mediated immune suppression may show an additive or synergistic effect. Partial responses were observed in a phase 1 trial of this agent in conjunction with radiation and temozolomide in patients with recurrent GBM [82][68]. Because PD-L1 is not frequently expressed on GBM [83[69][70],84], this strategy likely needs to be considered in the context of selected patients. In addition, the size of the therapeutic molecule requires consideration with respect to its ability to adequately cross the blood–brain barrier (BBB) at adequate concentrations to treat the tumor. Antisense nucleotides are another means for targeting TGF-β. These (AP12009) have been investigated in a non-randomized phase 2 trial in which they were directly administered into recurrent tumors using convection-enhanced delivery (CED). Partial and complete responses were observed [85][71]. There are a number of technical challenges currently associated with CED [86][72] which limit scalability and dampen the enthusiasm for later-stage clinical investigations.4.2. CSF-1
Colony-stimulating factor-1 (CSF-1) is a glycoprotein cytokine that functions through the receptor CSF1R [87][73] and regulates the differentiation of myeloid progenitors into dendritic cells, monocytes, and macrophages [88][74]. One of the most frequent immune cells within the TME are TAMs. The cells can become polarized to the M1 and M2 states [89,90][75][76] in which the M1 state exerts a pro-inflammatory, anti-tumor response [91][77] and the M2 state promotes tumor growth, invasion, metastasis, and resistance to therapy [92][78]. TAM-directed therapies using CSF-1 and CSF1R inhibitors have been tested in preclinical models of gliomas [93[79][80],94], as well as in clinical studies. A phase 2 trial (NCT01349036) of pexidartinib (PLX3397), a CSF1R inhibitor, in recurrent GBM was well tolerated but did not improve progression-free survival (PFS) [95][81]. Similarly, a combination of pexidartinib, radiation therapy, and temozolomide did not improve median PFS or OS in newly diagnosed GBM [96][82]. This lack of effect may be due to, at least in part, compensatory mechanisms such as CSF2-driven macrophage resistance or phosphatidylinositol 3-kinase [97][83].4.3. The Paradoxical Targeting of the Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) for Glioblastoma
GM-CSF is a hemopoietic growth factor and is responsible for the expansion and activation of macrophages and granulocytes [98][84]. GM-CSF modulates cell maturation proliferation and survival. GM-CSF boosts immune responses by promoting T and B cell expansion and differentiation and dendritic cell maturation, proliferation, and migration. It is from this immunological perspective that GM-CSF has been used in oncology clinical trials including a wide variety of peptide vaccine strategies for GBM patients. Notably, GM-CSF is elevated in cancer patients [99][85]. In glioblastoma, GM-CSF and its receptor can promote tumor progression likely through upregulating anti-apoptotic and pro-angiogenic signals via the activation of the signal transducer and activator of transcription 3 (STAT3) signaling pathway or by increasing the expression of VEGF and its receptor [100,101][86][87]. In the tumor environment, tumor cells, and tumor-associated microglial cells secrete GM-CSF [102,103,104][88][89][90]. Inhibiting GM-CSF thereby can suppress cancer cell growth and metastasis [103][89]. GM-CSF has been used in multiple large vaccine trials for GBM which could have had both beneficial and detrimental effects [1,72][1][58]. Given the dual pro-cancer and pro-inflammatory roles of GM-CSF, monotherapy inhibitors will likely not be tested in the context of glioma.5. Utilizing Anti-Tumoral Cytokines
5.1. Virus-Based Cytokine Expression
Virotherapy is an evolving class of immunotherapies based on the selective replication of these viruses in cancer cells to trigger tumor antigen presentation, immune activation, and subsequent tumor cytotoxicity [20,21,22][91][92][93]. Initiation and activation of apoptosis in the cancer cells and the induction of type I IFN is the underlying mechanism of these types of viruses. Viruses can also be devised to elaborate a variety of cytokines to modulate the immune system that thereby mediates the anti-tumor effect. The first oncolytic virus approved by the FDA in 2015 for the treatment of metastatic melanoma was talimogene laherparepvec (T-VEC), an engineered herpes simplex virus-1 that expresses human GM-CSF [105,106][94][95]. A series of preclinical studies have shown that cytokine-armed viruses can enhance immune response and provide additional survival benefits in glioma-bearing mice. For example, a virus expressing IL-4 prolonged survival in tumor-bearing mice [107][96] and one expressing a single-chain variable fragment of the epidermal growth factor receptor (EGFR) antibody conjugated to CCL5 increased the infiltration of innate and adaptive immune cells [108][97]. A number of cytokine-elaborating viruses have been tested in GBM [21,22,23,24][92][93][98][99] but tumor heterogeneity and the immune-suppressive TME have likely compromised clinical effectiveness thus far. Ad–RTS–hIL-12 is an adenoviral vector expressing IL-12 controlled by binding of an orally administered ligand, veledimex [62][48]. Safety, tolerability, and feasibility were demonstrated in a phase 1 monotherapy trial in recurrent high-grade glioma. The ability to measure extra-CNS spill-over of IL-12 and its downstream product IFN-γ was demonstrated via elevated serum concentrations. Based on preclinical studies, the intracranial concentration of cytokines was likely substantially higher than what could be measured in the serum. Post-treatment resected tumor tissue demonstrated an increase in T cell infiltration of the tumor. This approach has been further investigated in conjunction with PD-1 blockade in the phase 1 [61][47] and phase 2 settings [109][100]. As discussed earlier, the highest level of IL-12 production did not appear to be the optimal dose for impacting survival and in turn was not utilized as the phase 2 dose. Two other IL-12-based viral vector gene therapy approaches are currently under investigation in gliomas. Ad-TD-nsIL12, a human adenovirus with three genes deleted and expressing human non-secretory IL-12, was developed to minimize IL-12 toxic effects [110][101]. A phase I Ad-TD-nsIL12 trial (NCT05717699, NCT05717712) in pediatric patients with diffuse intrinsic pontine glioma is currently recruiting patients in China. Another phase 1/2 trial (NSC 733972) is now enrolling patients with high-grade gliomas to study the combination of M032, a genetically engineered HSV-1 expressing IL-12, with pembrolizumab.5.2. The Addition of IFN-α with the Standard of Care Temozolomide
IFN-α can inhibit tumor cell proliferation, enhance the cytotoxic activity of macrophages and natural killer (NK) cells, and prevent the formation of blood vessels in tumors [111][102]. A multi-center randomized phase 3 clinical trial enrolled 199 patients with high-grade gliomas. After receiving standard radiation therapy with concurrent temozolomide, patients were randomized to receive either temozolomide or temozolomide with IFN-α. The median OS of patients in the temozolomide plus IFN-α group was 26.7 months, which was longer than that in the standard of care group of 18.8 months (p = 0.005). Seizure and influenza-like symptoms were more common in the combination group [48][34]. The potential benefit was consistent with a prior study that demonstrated that a pegylated formulation had some benefit in addition to temozolomide [112][103]. However, a prior phase III study of 275 randomized high-grade glioma patients had demonstrated that IFN-α did not improve time to disease progression or OS when added to treatment with radiation therapy and carmustine. Patients treated with IFN-α experienced more fevers, chills, myalgia, somnolence, confusion, and neurological deficits [49][35]. The differences in outcomes between these trials may have been a function of the combination with the type of chemotherapy.5.3. Systemic Cytokine Therapy in Conjunction with Brain Tumor Vaccines
The objective of cancer vaccines is to stimulate adaptive immunity against tumor antigens to control tumor growth [113][104]. The first cancer vaccine approved by the FDA was sipuleucel-T (Provenge), which is a personalized vaccine developed using ex vivo activated peripheral-blood mononuclear cells co-incubated with a recombinant fusion protein (PA2024) to control asymptomatic metastatic castration-resistant prostate cancer [114][105]. Various types of GBM vaccines have been developed that are usually administered in conjunction with GM-CSF [71,115,116,117,118,119,120,121,122,123][57][106][107][108][109][110][111][112][113][114]. Thus far, they have not demonstrated an improvement in survival. Newer strategies involve the co-administration of additional cytokines to augment the potential activity of glioma vaccines. For example, IL-12 was shown to improve the therapeutic efficacy in preclinical murine models bearing intracranial gliomas treated with dendritic cells loaded with GL261 mRNA [124][115]. Several different approaches are being investigated with all appearing safe and having acceptable tolerability thus far.5.4. Cell-Based Therapies
Cell-based therapies rely on genetically modified immune cells such as T, NK, and B cells. Adoptive transfer of genetically engineered chimeric antigen receptor (CAR) T cells demonstrated success in hematologic malignancies and melanoma with six CAR T cell therapies having received FDA approval [125][116]. While preclinical studies of CAR T therapy were effective in brain tumor control [126[117][118],127], overall response rates have been low, likely because of antigen heterogeneity [128,129][119][120] and the immune-suppressive TME [130,131][121][122]. CAR T therapy may have the ability to reprogram TME and thus may be a compelling partnering approach with other treatment modalities [131][122]. Improving CAR T therapy can be achieved by engineered expression of cytokines or their receptors to enhance T cell activation, proliferation, and trafficking. In preclinical testing, disialoganglioside (GD2)-targeting CARs engineered with constitutively active IL-7 receptor or IL-15, enhanced survival in GBM xenograft models [132,133][123][124]. In another approach, the expression of CXCR1 or CXCR2 in CAR T cells improved trafficking in a GBM model [134][125]. An upcoming phase 1 trial (NCT05353530) has been designed to assess the safety and feasibility of IL-8 receptor-modified CD70 CAR T treatment in CD70+ and MGMT-unmethylated GBM patients. IL13 receptor alpha 2 (IL13Ra2) is a monomeric receptor of IL-13 [135][126] that is expressed in ~70% of GBM patients. IL-13Ra2 is associated with higher-grade glioma and poor prognosis [136][127]. Data from the clinical experience of IL-13Ra2 CAR T intracranial administration supported the safety of CAR T in patients with recurrent GBM [137][128]. NK cells have also been evaluated in the treatment of gliomas [138,139][129][130]. NK cells, a key component of innate immunity, facilitate cell lysis by degranulation achieved by the activating receptor NK group 2 member D (NKG2D) [140][131], killer cell immunoglobulin-like receptor (KIR), and coactivating/adhesion DNAX-activating molecule (DNAM-1) [141][132]. Because NK cells become deactivated by TGF-β in the immune-suppressive TME of GBM [138][129], these cells are co-administered with IL-2 and a TGF-βR1 inhibitor (NCT05400122) or are genetically modified so that the TGF-βR is deleted (NCT04991870) in ongoing clinical trials for colorectal adenocarcinoma and GBM patients, respectively.5.5. Cytokines Associated with Toxicity in GLIOMA Patients
Distinct elevated serum cytokines may be associated with side effects in glioma patients. In one study, plasma profiling of patients treated with the antiangiogenic agent aflibercept in 28 patients with recurrent GBM revealed that changes in IL-13 from baseline to 24 h predicted on-target toxicities. Increases in IL-1β, IL-6, and IL-10 at 24 h were significantly associated with fatigue [142][133].5.6. The Modern Era of Monitoring Intratumoral Cytokines
Under most circumstances, cytokines of CNS tumor patients are measured in the periphery, and these are likely not fully representative of intra-CNS, including intratumoral, concentrations. To determine both the absolute intratumoral concentrations and to follow the longitudinal kinetics, microdialysis catheters can be implanted with minimal risk [143][134]. This type of analysis is important since it may also identify those subjects that are showing early signs of response, whereas those who do not demonstrate immune effector responses could be spared further ineffective therapy or an alternative therapy based on the changes in the tumor microenvironment. This is contingent on the conditions that cytokines alone would be biologically meaningful as a biomarker of response and that the captured time point for analysis coincides with the therapeutic monitoring period.5.7. Modulating Cytokines in Glioma Preclinical Model
There are substantial preclinical efforts to use cytokines, especially in adoptive cellular strategies. For example, IL-7 expressed by CAR T improved the survival outcome in a GBM murine model [144][135]. In another model, IL-15-modified CAR T also improved median survival [127][118]. Thus far, it is unclear in what specific contexts these cytokine modifications of CAR T cells should be optimally used, the prioritization of which ones, or the combinations. A key limitation is the distribution of adaptive immune therapies through a complex heterogeneous TME. In addition to the delivery of cytokines using viral vectors, an alternative strategy would be the deposition of cells elaborating cytokines and/or chemokines in the TME using BBB opening ultrasound [26][136]. This type of strategy allows for large molecules to be deposited into the glioblastoma TME. Researchers' group engineered antigen-presenting cells to express CXCL10. These were deposited into the TME of gliomas and markedly increased the number of T cells in the TME and increased median survival [145][137]. Moving forward, one could engineer off-the-shelf cells that have been transduced with a variety of pro-inflammatory cytokines that are deposited into the TME using BBB opening ultrasound for sustained delivery.References
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385.
- Lukas, R.V.; Wainwright, D.A.; Ladomersky, E.; Sachdev, S.; Sonabend, A.M.; Stupp, R. Newly Diagnosed Glioblastoma: A Review on Clinical Management. Oncology 2019, 33, 91–100.
- Lukas, R.V.; Mrugala, M.M. Pivotal therapeutic trials for infiltrating gliomas and how they affect clinical practice. Neurooncol. Pract. 2017, 4, 209–219.
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316.
- Ellingson, B.M.; Wen, P.Y.; Chang, S.M.; van den Bent, M.; Vogelbaum, M.A.; Li, G.; Li, S.; Kim, J.; Youssef, G.; Wick, W.; et al. Objective response rate (ORR) targets for recurrent glioblastoma clinical trials based on the historic association between ORR and median overall survival. Neuro-Oncology 2023, 25, 1017–1028.
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavare, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014.
- Cui, X.; Ma, C.; Vasudevaraja, V.; Serrano, J.; Tong, J.; Peng, Y.; Delorenzo, M.; Shen, G.; Frenster, J.; Morales, R.T.; et al. Dissecting the immunosuppressive tumor microenvironments in Glioblastoma-on-a-Chip for optimized PD-1 immunotherapy. eLife 2020, 9, e52253.
- Drumm, M.R.; Dixit, K.S.; Grimm, S.; Kumthekar, P.; Lukas, R.V.; Raizer, J.J.; Stupp, R.; Chheda, M.G.; Kam, K.L.; McCord, M.; et al. Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas. Neuro-Oncology 2020, 22, 470–479.
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499.
- Zhang, D.Y.; Singer, L.; Sonabend, A.M.; Lukas, R.V. Gene Therapy for the Treatment of Malignant Glioma. Adv. Oncol. 2021, 1, 189–202.
- Waldmann, T.A. Cytokines in Cancer Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028472.
- Yang, F.; He, Z.; Duan, H.; Zhang, D.; Li, J.; Yang, H.; Dorsey, J.F.; Zou, W.; Nabavizadeh, S.A.; Bagley, S.J.; et al. Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40. Nat. Commun. 2021, 12, 3424.
- Birocchi, F.; Cusimano, M.; Rossari, F.; Beretta, S.; Rancoita, P.M.V.; Ranghetti, A.; Colombo, S.; Costa, B.; Angel, P.; Sanvito, F.; et al. Targeted inducible delivery of immunoactivating cytokines reprograms glioblastoma microenvironment and inhibits growth in mouse models. Sci. Transl. Med. 2022, 14, eabl4106.
- Dahlberg, D.; Rummel, J.; Distante, S.; De Souza, G.A.; Stensland, M.E.; Mariussen, E.; Rootwelt, H.; Voie, O.; Hassel, B. Glioblastoma microenvironment contains multiple hormonal and non-hormonal growth-stimulating factors. Fluids Barriers CNS 2022, 19, 45.
- Wu, A.; Wei, J.; Kong, L.Y.; Wang, Y.; Priebe, W.; Qiao, W.; Sawaya, R.; Heimberger, A.B. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncology 2010, 12, 1113–1125.
- Morandi, F.; Fainardi, E.; Rizzo, R.; Rouas-Freiss, N. The role of HLA-class Ib molecules in immune-related diseases, tumors, and infections. J. Immunol. Res. 2014, 2014, 231618.
- Kohanbash, G.; Okada, H. Myeloid-derived suppressor cells (MDSCs) in gliomas and glioma-development. Immunol. Investig. 2012, 41, 658–679.
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150.
- Raber, P.L.; Thevenot, P.; Sierra, R.; Wyczechowska, D.; Halle, D.; Ramirez, M.E.; Ochoa, A.C.; Fletcher, M.; Velasco, C.; Wilk, A.; et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int. J. Cancer 2014, 134, 2853–2864.
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009, 182, 4499–4506.
- Rodriguez, P.C.; Ochoa, A.C.; Al-Khami, A.A. Arginine Metabolism in Myeloid Cells Shapes Innate and Adaptive Immunity. Front. Immunol. 2017, 8, 93.
- Zhang, Z.; Huang, X.; Li, J.; Fan, H.; Yang, F.; Zhang, R.; Yang, Y.; Feng, S.; He, D.; Sun, W.; et al. Interleukin 10 promotes growth and invasion of glioma cells by up-regulating KPNA 2 in vitro. J. Cancer Res. Ther. 2019, 15, 927–932.
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β 1. J. Immunol. 2009, 182, 240–249.
- Dumas, A.A.; Pomella, N.; Rosser, G.; Guglielmi, L.; Vinel, C.; Millner, T.O.; Rees, J.; Aley, N.; Sheer, D.; Wei, J.; et al. Microglia promote glioblastoma via mTOR-mediated immunosuppression of the tumour microenvironment. EMBO J. 2020, 39, e103790.
- Zheng, S.; Hedl, M.; Abraham, C. TAM receptor-dependent regulation of SOCS3 and MAPKs contributes to proinflammatory cytokine downregulation following chronic NOD2 stimulation of human macrophages. J. Immunol. 2015, 194, 1928–1937.
- Dannenmann, S.R.; Thielicke, J.; Stockli, M.; Matter, C.; von Boehmer, L.; Cecconi, V.; Hermanns, T.; Hefermehl, L.; Schraml, P.; Moch, H.; et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology 2013, 2, e23562.
- Chongsathidkiet, P.; Jackson, C.; Koyama, S.; Loebel, F.; Cui, X.; Farber, S.H.; Woroniecka, K.; Elsamadicy, A.A.; Dechant, C.A.; Kemeny, H.R.; et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 2018, 24, 1459–1468.
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186.
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2274–2279.
- Lucey, D.R.; Clerici, M.; Shearer, G.M. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 1996, 9, 532–562.
- Aziz, N.; Detels, R.; Quint, J.J.; Li, Q.; Gjertson, D.; Butch, A.W. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 2016, 84, 17–24.
- Ozaki, K.; Leonard, W.J. Cytokine and cytokine receptor pleiotropy and redundancy. J. Biol. Chem. 2002, 277, 29355–29358.
- Floros, T.; Tarhini, A.A. Anticancer Cytokines: Biology and Clinical Effects of Interferon-alpha2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin. Oncol. 2015, 42, 539–548.
- Guo, C.; Yang, Q.; Xu, P.; Deng, M.; Jiang, T.; Cai, L.; Li, J.; Sai, K.; Xi, S.; Ouyang, H.; et al. Adjuvant Temozolomide Chemotherapy With or Without Interferon Alfa Among Patients with Newly Diagnosed High-grade Gliomas: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2253285.
- Buckner, J.C.; Schomberg, P.J.; McGinnis, W.L.; Cascino, T.L.; Scheithauer, B.W.; O’Fallon, J.R.; Morton, R.F.; Kuross, S.A.; Mailliard, J.A.; Hatfield, A.K.; et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer 2001, 92, 420–433.
- Brandes, A.A.; Scelzi, E.; Zampieri, P.; Rigon, A.; Rotilio, A.; Amista, P.; Berti, F.; Fiorentino, M.V. Phase II trial with BCNU plus alpha-interferon in patients with recurrent high-grade gliomas. Am. J. Clin. Oncol. 1997, 20, 364–367.
- Buckner, J.C.; Brown, L.D.; Kugler, J.W.; Cascino, T.L.; Krook, J.E.; Mailliard, J.A.; Kardinal, C.G.; Tschetter, L.K.; O’Fallon, J.R.; Scheithauer, B.W. Phase II evaluation of recombinant interferon alpha and BCNU in recurrent glioma. J. Neurosurg. 1995, 82, 430–435.
- Dillman, R.O.; Shea, W.M.; Tai, D.F.; Mahdavi, K.; Barth, N.M.; Kharkar, B.R.; Poor, M.M.; Church, C.K.; DePriest, C. Interferon-alpha2a and 13-cis-retinoic acid with radiation treatment for high-grade glioma. Neuro-Oncology 2001, 3, 35–41.
- Warren, K.; Bent, R.; Wolters, P.L.; Prager, A.; Hanson, R.; Packer, R.; Shih, J.; Camphausen, K. A phase 2 study of pegylated interferon α-2b (PEG-Intron(®)) in children with diffuse intrinsic pontine glioma. Cancer 2012, 118, 3607–3613.
- Wakabayashi, T.; Natsume, A.; Mizusawa, J.; Katayama, H.; Fukuda, H.; Sumi, M.; Nishikawa, R.; Narita, Y.; Muragaki, Y.; Maruyama, T.; et al. JCOG0911 INTEGRA study: A randomized screening phase II trial of interferonβ plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma. J. Neurooncol. 2018, 138, 627–636.
- Wakabayashi, T.; Kayama, T.; Nishikawa, R.; Takahashi, H.; Hashimoto, N.; Takahashi, J.; Aoki, T.; Sugiyama, K.; Ogura, M.; Natsume, A.; et al. A multicenter phase I trial of combination therapy with interferon-β and temozolomide for high-grade gliomas (INTEGRA study): The final report. J. Neurooncol. 2011, 104, 573–577.
- Fine, H.A.; Wen, P.Y.; Robertson, M.; O’Neill, A.; Kowal, J.; Loeffler, J.S.; Black, P.M. A phase I trial of a new recombinant human beta-interferon (BG9015) for the treatment of patients with recurrent gliomas. Clin. Cancer Res. 1997, 3, 381–387.
- Packer, R.J.; Prados, M.; Phillips, P.; Nicholson, H.S.; Boyett, J.M.; Goldwein, J.; Rorke, L.B.; Needle, M.N.; Sutton, L.; Zimmerman, R.A.; et al. Treatment of children with newly diagnosed brain stem gliomas with intravenous recombinant beta-interferon and hyperfractionated radiation therapy: A childrens cancer group phase I/II study. Cancer 1996, 77, 2150–2156.
- Allen, J.; Packer, R.; Bleyer, A.; Zeltzer, P.; Prados, M.; Nirenberg, A. Recombinant interferon beta: A phase I-II trial in children with recurrent brain tumors. J. Clin. Oncol. 1991, 9, 783–788.
- Lillehei, K.O.; Mitchell, D.H.; Johnson, S.D.; McCleary, E.L.; Kruse, C.A. Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery 1991, 28, 16–23.
- Wolff, J.E.; Wagner, S.; Reinert, C.; Gnekow, A.; Kortmann, R.D.; Kuhl, J.; Van Gool, S.W. Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J. Neurooncol. 2006, 79, 315–321.
- Chiocca, E.A.; Gelb, A.B.; Chen, C.C.; Rao, G.; Reardon, D.A.; Wen, P.Y.; Bi, W.L.; Peruzzi, P.; Amidei, C.; Triggs, D.; et al. Combined immunotherapy with controlled interleukin-12 gene therapy and immune checkpoint blockade in recurrent glioblastoma: An open-label, multi-institutional phase I trial. Neuro-Oncology 2022, 24, 951–963.
- Chiocca, E.A.; Yu, J.S.; Lukas, R.V.; Solomon, I.H.; Ligon, K.L.; Nakashima, H.; Triggs, D.A.; Reardon, D.A.; Wen, P.; Stopa, B.M.; et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 2019, 11, eaaw5680.
- Patel, D.M.; Foreman, P.M.; Nabors, L.B.; Riley, K.O.; Gillespie, G.Y.; Markert, J.M. Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016, 27, 69–78.
- Lee, E.Q.; Duda, D.G.; Muzikansky, A.; Gerstner, E.R.; Kuhn, J.G.; Reardon, D.A.; Nayak, L.; Norden, A.D.; Doherty, L.; LaFrankie, D.; et al. Phase I and Biomarker Study of Plerixafor and Bevacizumab in Recurrent High-Grade Glioma. Clin. Cancer Res. 2018, 24, 4643–4649.
- Butowski, N.; Colman, H.; De Groot, J.F.; Omuro, A.M.; Nayak, L.; Wen, P.Y.; Cloughesy, T.F.; Marimuthu, A.; Haidar, S.; Perry, A.; et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: An Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncology 2016, 18, 557–564.
- Capper, D.; von Deimling, A.; Brandes, A.A.; Carpentier, A.F.; Kesari, S.; Sepulveda-Sanchez, J.M.; Wheeler, H.R.; Chinot, O.; Cher, L.; Steinbach, J.P.; et al. Biomarker and Histopathology Evaluation of Patients with Recurrent Glioblastoma Treated with Galunisertib, Lomustine, or the Combination of Galunisertib and Lomustine. Int. J. Mol. Sci. 2017, 18, 995.
- Rodon, J.; Carducci, M.; Sepulveda-Sanchez, J.M.; Azaro, A.; Calvo, E.; Seoane, J.; Brana, I.; Sicart, E.; Gueorguieva, I.; Cleverly, A.; et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Investig. New Drugs 2015, 33, 357–370.
- Bogdahn, U.; Hau, P.; Stockhammer, G.; Venkataramana, N.K.; Mahapatra, A.K.; Suri, A.; Balasubramaniam, A.; Nair, S.; Oliushine, V.; Parfenov, V.; et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro-Oncology 2011, 13, 132–142.
- Oshiro, S.; Tsugu, H.; Komatsu, F.; Ohnishi, H.; Ueno, Y.; Sakamoto, S.; Fukushima, T.; Soma, G. Evaluation of intratumoral administration of tumor necrosis factor-alpha in patients with malignant glioma. Anticancer. Res. 2006, 26, 4027–4032.
- Colman, H.; Berkey, B.A.; Maor, M.H.; Groves, M.D.; Schultz, C.J.; Vermeulen, S.; Nelson, D.F.; Mehta, M.P.; Yung, W.K.; Radiation Therapy Oncology, G. Phase II Radiation Therapy Oncology Group trial of conventional radiation therapy followed by treatment with recombinant interferon-beta for supratentorial glioblastoma: Results of RTOG 9710. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 818–824.
- Curry, W.T., Jr.; Gorrepati, R.; Piesche, M.; Sasada, T.; Agarwalla, P.; Jones, P.S.; Gerstner, E.R.; Golby, A.J.; Batchelor, T.T.; Wen, P.Y.; et al. Vaccination with Irradiated Autologous Tumor Cells Mixed with Irradiated GM-K562 Cells Stimulates Antitumor Immunity and T Lymphocyte Activation in Patients with Recurrent Malignant Glioma. Clin. Cancer Res. 2016, 22, 2885–2896.
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin. Cancer Res. 2020, 26, 1586–1594.
- Sankhla, S.K.; Nadkarni, J.S.; Bhagwati, S.N. Adoptive immunotherapy using lymphokine-activated killer (LAK) cells and interleukin-2 for recurrent malignant primary brain tumors. J. Neurooncol. 1996, 27, 133–140.
- Boiardi, A.; Silvani, A.; Ruffini, P.A.; Rivoltini, L.; Parmiani, G.; Broggi, G.; Salmaggi, A. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol. Immunother. 1994, 39, 193–197.
- Merchant, R.E.; McVicar, D.W.; Merchant, L.H.; Young, H.F. Treatment of recurrent malignant glioma by repeated intracerebral injections of human recombinant interleukin-2 alone or in combination with systemic interferon-alpha. Results of a phase I clinical trial. J. Neurooncol. 1992, 12, 75–83.
- Jacobs, S.K.; Wilson, D.J.; Kornblith, P.L.; Grimm, E.A. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: Phase I trial. Cancer Res. 1986, 46, 2101–2104.
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940.
- Matsuda, S.; Revandkar, A.; Dubash, T.D.; Ravi, A.; Wittner, B.S.; Lin, M.; Morris, R.; Burr, R.; Guo, H.; Seeger, K.; et al. TGF-β in the microenvironment induces a physiologically occurring immune-suppressive senescent state. Cell Rep. 2023, 42, 112129.
- Chao, M.; Liu, N.; Sun, Z.; Jiang, Y.; Jiang, T.; Xv, M.; Jia, L.; Tu, Y.; Wang, L. TGF-β Signaling Promotes Glioma Progression Through Stabilizing Sox9. Front. Immunol. 2020, 11, 592080.
- Wick, A.; Desjardins, A.; Suarez, C.; Forsyth, P.; Gueorguieva, I.; Burkholder, T.; Cleverly, A.L.; Estrem, S.T.; Wang, S.; Lahn, M.M.; et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Investig. New Drugs 2020, 38, 1570–1579.
- Brandes, A.A.; Carpentier, A.F.; Kesari, S.; Sepulveda-Sanchez, J.M.; Wheeler, H.R.; Chinot, O.; Cher, L.; Steinbach, J.P.; Capper, D.; Specenier, P.; et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro-Oncology 2016, 18, 1146–1156.
- Khasraw, M.; Weller, M.; Lorente, D.; Kolibaba, K.; Lee, C.K.; Gedye, C.; de La Fuente, M.I.; Vicente, D.; Reardon, D.A.; Gan, H.K.; et al. Bintrafusp alfa (M7824), a bifunctional fusion protein targeting TGF-β and PD-L1: Results from a phase I expansion cohort in patients with recurrent glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab058.
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncology 2016, 18, 195–205.
- Hodges, T.R.; Ott, M.; Xiu, J.; Gatalica, Z.; Swensen, J.; Zhou, S.; Huse, J.T.; de Groot, J.; Li, S.; Overwijk, W.W.; et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro-Oncology 2017, 19, 1047–1057.
- Uckun, F.M.; Qazi, S.; Hwang, L.; Trieu, V.N. Recurrent or Refractory High-Grade Gliomas Treated by Convection-Enhanced Delivery of a TGFbeta2-Targeting RNA Therapeutic: A Post-Hoc Analysis with Long-Term Follow-Up. Cancers 2019, 11, 1892.
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706.
- Hume, D.A.; MacDonald, K.P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810–1820.
- Rojo, R.; Raper, A.; Ozdemir, D.D.; Lefevre, L.; Grabert, K.; Wollscheid-Lengeling, E.; Bradford, B.; Caruso, M.; Gazova, I.; Sanchez, A.; et al. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat. Commun. 2019, 10, 3215.
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2019, 9, 1512.
- Wu, M.; Wu, L.; Wu, W.; Zhu, M.; Li, J.; Wang, Z.; Li, J.; Ding, R.; Liang, Y.; Li, L.; et al. Phagocytosis of Glioma Cells Enhances the Immunosuppressive Phenotype of Bone Marrow-Derived Macrophages. Cancer Res. 2023, 83, 771–785.
- Wang, F.; Zhang, S.; Jeon, R.; Vuckovic, I.; Jiang, X.; Lerman, A.; Folmes, C.D.; Dzeja, P.D.; Herrmann, J. Interferon Gamma Induces Reversible Metabolic Reprogramming of M1 Macrophages to Sustain Cell Viability and Pro-Inflammatory Activity. eBioMedicine 2018, 30, 303–316.
- Vidyarthi, A.; Agnihotri, T.; Khan, N.; Singh, S.; Tewari, M.K.; Radotra, B.D.; Chatterjee, D.; Agrewala, J.N. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol. Immunother. 2019, 68, 1995–2004.
- Akkari, L.; Bowman, R.L.; Tessier, J.; Klemm, F.; Handgraaf, S.M.; de Groot, M.; Quail, D.F.; Tillard, L.; Gadiot, J.; Huse, J.T.; et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 2020, 12, eaaw7843.
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272.
- Butowski, N.A.; Colman, H.; Groot, J.F.D.; Omuro, A.M.P.; Nayak, L.; Cloughesy, T.F.; Marimuthu, A.; Perry, A.; Phillips, J.J.; West, B.; et al. A phase 2 study of orally administered PLX3397 in patients with recurrent glioblastoma. J. Clin. Oncol. 2014, 32, 2023.
- Colman, H.; Raizer, J.J.; Walbert, T.; Plotkin, S.R.; Chamberlain, M.C.; Wong, E.T.; Puduvalli, V.K.; Reardon, D.A.; Iwamoto, F.M.; Mrugala, M.M.; et al. Phase 1b/2 study of pexidartinib (PEX) in combination with radiation therapy (XRT) and temozolomide (TMZ) in newly diagnosed glioblastoma. J. Clin. Oncol. 2018, 36, 2015.
- Klemm, F.; Mockl, A.; Salamero-Boix, A.; Alekseeva, T.; Schaffer, A.; Schulz, M.; Niesel, K.; Maas, R.R.; Groth, M.; Elie, B.T.; et al. Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nat. Cancer 2021, 2, 1086–1101.
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265.
- Albulescu, R.; Codrici, E.; Popescu, I.D.; Mihai, S.; Necula, L.G.; Petrescu, D.; Teodoru, M.; Tanase, C.P. Cytokine patterns in brain tumour progression. Mediat. Inflamm. 2013, 2013, 979748.
- Jung, K.H.; Chu, K.; Lee, S.T.; Kim, S.J.; Sinn, D.I.; Kim, S.U.; Kim, M.; Roh, J.K. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res. 2006, 1073–1074, 190–201.
- Ohki, Y.; Heissig, B.; Sato, Y.; Akiyama, H.; Zhu, Z.; Hicklin, D.J.; Shimada, K.; Ogawa, H.; Daida, H.; Hattori, K.; et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005, 19, 2005–2007.
- Gabrusiewicz, K.; Ellert-Miklaszewska, A.; Lipko, M.; Sielska, M.; Frankowska, M.; Kaminska, B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE 2011, 6, e23902.
- Curran, C.S.; Evans, M.D.; Bertics, P.J. GM-CSF production by glioblastoma cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. J. Immunol. 2011, 187, 1254–1263.
- Kucerova, L.; Matuskova, M.; Hlubinova, K.; Altanerova, V.; Altaner, C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol. Cancer 2010, 9, 129.
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bähr, O.; et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro-Oncology 2023, 25, 123–134.
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114.
- Tobias, A.L.; Thaci, B.; Auffinger, B.; Rincon, E.; Balyasnikova, I.V.; Kim, C.K.; Han, Y.; Zhang, L.; Aboody, K.S.; Ahmed, A.U.; et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013, 2, 655–666.
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.; Berger, A.C.; Conry, R.; Demidov, L.; Sharma, A.; Treichel, S.A.; Radcliffe, H.; Gorski, K.S.; et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: A randomized, open-label, phase 2 trial. Nat. Med. 2021, 27, 1789–1796.
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788.
- Andreansky, S.; He, B.; van Cott, J.; McGhee, J.; Markert, J.M.; Gillespie, G.Y.; Roizman, B.; Whitley, R.J. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998, 5, 121–130.
- Tian, L.; Xu, B.; Chen, Y.; Li, Z.; Wang, J.; Zhang, J.; Ma, R.; Cao, S.; Hu, W.; Chiocca, E.A.; et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity. Nat. Cancer 2022, 3, 1318–1335.
- Gesundheit, B.; Ben-David, E.; Posen, Y.; Ellis, R.; Wollmann, G.; Schneider, E.M.; Aigner, K.; Brauns, L.; Nesselhut, T.; Ackva, I.; et al. Effective Treatment of Glioblastoma Multiforme With Oncolytic Virotherapy: A Case-Series. Front. Oncol. 2020, 10, 702.
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 2022, 13, 4119.
- Lukas, R.; Oberheim-Bush, N.A.; Cavaliere, R.; Landolfi, J.; Yu, J.S.; Chen, C.; Cordova, C.; Amidei, C.; Buck, J.Y.; Hadar, N.; et al. CTIM-20. Final Results of Controlled IL-12 Monotherapy and in Combination with PD-1 Inhibitor in Adult Subjects with Recurrent Glioblastoma. Neuro-Oncology 2021, 23, vi54.
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395.
- Vidal, P. Interferon α in cancer immunoediting: From elimination to escape. Scand. J. Immunol. 2020, 91, e12863.
- Groves, M.D.; Puduvalli, V.K.; Gilbert, M.R.; Levin, V.A.; Conrad, C.A.; Liu, V.H.; Hunter, K.; Meyers, C.; Hess, K.R.; Alfred Yung, W.K. Two phase II trials of temozolomide with interferon-alpha2b (pegylated and non-pegylated) in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2009, 101, 615–620.
- Ott, P.A.; Wu, C.J. Cancer Vaccines: Steering T Cells Down the Right Path to Eradicate Tumors. Cancer Discov. 2019, 9, 476–481.
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006, 24, 3089–3094.
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023, 9, 112–121.
- Ahluwalia, M.S.; Reardon, D.A.; Abad, A.P.; Curry, W.T.; Wong, E.T.; Figel, S.A.; Mechtler, L.L.; Peereboom, D.M.; Hutson, A.D.; Withers, H.G.; et al. Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2023, 41, 1453–1465.
- Bota, D.A.; Taylor, T.H.; Piccioni, D.E.; Duma, C.M.; LaRocca, R.V.; Kesari, S.; Carrillo, J.A.; Abedi, M.; Aiken, R.D.; Hsu, F.P.K.; et al. Phase 2 study of AV-GBM-1 (a tumor-initiating cell targeted dendritic cell vaccine) in newly diagnosed Glioblastoma patients: Safety and efficacy assessment. J. Exp. Clin. Cancer Res. 2022, 41, 344.
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245.
- Rampling, R.; Peoples, S.; Mulholland, P.J.; James, A.; Al-Salihi, O.; Twelves, C.J.; McBain, C.; Jefferies, S.; Jackson, A.; Stewart, W.; et al. A Cancer Research UK First Time in Human Phase I Trial of IMA950 (Novel Multipeptide Therapeutic Vaccine) in Patients with Newly Diagnosed Glioblastoma. Clin. Cancer Res. 2016, 22, 4776–4785.
- Heimberger, A.B.; Crotty, L.E.; Archer, G.E.; Hess, K.R.; Wikstrand, C.J.; Friedman, A.H.; Friedman, H.S.; Bigner, D.D.; Sampson, J.H. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin. Cancer Res. 2003, 9, 4247–4254.
- Sampson, J.H.; Archer, G.E.; Mitchell, D.A.; Heimberger, A.B.; Herndon, J.E., 2nd; Lally-Goss, D.; McGehee-Norman, S.; Paolino, A.; Reardon, D.A.; Friedman, A.H.; et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol. Cancer Ther. 2009, 8, 2773–2779.
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E., 2nd; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729.
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro-Oncology 2015, 17, 854–861.
- Ciesielski, M.J.; Apfel, L.; Barone, T.A.; Castro, C.A.; Weiss, T.C.; Fenstermaker, R.A. Antitumor effects of a xenogeneic survivin bone marrow derived dendritic cell vaccine against murine GL261 gliomas. Cancer Immunol. Immunother. 2006, 55, 1491–1503.
- Sadelain, M.; Riviere, I.; Riddell, S. Therapeutic T cell engineering. Nature 2017, 545, 423–431.
- Pituch, K.C.; Miska, J.; Krenciute, G.; Panek, W.K.; Li, G.; Rodriguez-Cruz, T.; Wu, M.; Han, Y.; Lesniak, M.S.; Gottschalk, S.; et al. Adoptive Transfer of IL13Ralpha2-Specific Chimeric Antigen Receptor T Cells Creates a Pro-inflammatory Environment in Glioblastoma. Mol. Ther. 2018, 26, 986–995.
- Zannikou, M.; Duffy, J.T.; Levine, R.N.; Seblani, M.; Liu, Q.; Presser, A.; Arrieta, V.A.; Chen, C.J.; Sonabend, A.M.; Horbinski, C.M.; et al. IL15 modification enables CAR T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in GBM. J. Immunother. Cancer 2023, 11, e006239.
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984.
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569.
- Chae, M.; Peterson, T.E.; Balgeman, A.; Chen, S.; Zhang, L.; Renner, D.N.; Johnson, A.J.; Parney, I.F. Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model. Neuro-Oncology 2015, 17, 978–991.
- Ott, M.; Prins, R.M.; Heimberger, A.B. The immune landscape of common CNS malignancies: Implications for immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 729–744.
- Shum, T.; Omer, B.; Tashiro, H.; Kruse, R.L.; Wagner, D.L.; Parikh, K.; Yi, Z.; Sauer, T.; Liu, D.; Parihar, R.; et al. Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells. Cancer Discov. 2017, 7, 1238–1247.
- Gargett, T.; Ebert, L.M.; Truong, N.T.H.; Kollis, P.M.; Sedivakova, K.; Yu, W.; Yeo, E.C.F.; Wittwer, N.L.; Gliddon, B.L.; Tea, M.N.; et al. GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J. Immunother. Cancer 2022, 10, e005187.
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 2019, 10, 4016.
- Lupardus, P.J.; Birnbaum, M.E.; Garcia, K.C. Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Structure 2010, 18, 332–342.
- Brown, C.E.; Warden, C.D.; Starr, R.; Deng, X.; Badie, B.; Yuan, Y.C.; Forman, S.J.; Barish, M.E. Glioma IL13Ralpha2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE 2013, 8, e77769.
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072.
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the alphav integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021, 131, e142116.
- Mitwasi, N.; Feldmann, A.; Arndt, C.; Koristka, S.; Berndt, N.; Jureczek, J.; Loureiro, L.R.; Bergmann, R.; Mathe, D.; Hegedus, N.; et al. “UniCAR”-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci. Rep. 2020, 10, 2141.
- Raulet, D.H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003, 3, 781–790.
- Sayitoglu, E.C.; Georgoudaki, A.M.; Chrobok, M.; Ozkazanc, D.; Josey, B.J.; Arif, M.; Kusser, K.; Hartman, M.; Chinn, T.M.; Potens, R.; et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020, 11, 40.
- Shonka, N.; Piao, Y.; Gilbert, M.; Yung, A.; Chang, S.; DeAngelis, L.M.; Lassman, A.B.; Liu, J.; Cloughesy, T.; Robins, H.I.; et al. Cytokines associated with toxicity in the treatment of recurrent glioblastoma with aflibercept. Target. Oncol. 2013, 8, 117–125.
- Lynes, J.; Jackson, S.; Sanchez, V.; Dominah, G.; Wang, X.; Kuek, A.; Hayes, C.P.; Benzo, S.; Scott, G.C.; Chittiboina, P.; et al. Cytokine Microdialysis for Real-Time Immune Monitoring in Glioblastoma Patients Undergoing Checkpoint Blockade. Neurosurgery 2019, 84, 945–953.
- Swan, S.L.; Mehta, N.; Ilich, E.; Shen, S.H.; Wilkinson, D.S.; Anderson, A.R.; Segura, T.; Sanchez-Perez, L.; Sampson, J.H.; Bellamkonda, R.V. IL7 and IL7 Flt3L co-expressing CAR T cells improve therapeutic efficacy in mouse EGFRvIII heterogeneous glioblastoma. Front. Immunol. 2023, 14, 1085547.
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378.
- Sabbagh, A.; Beccaria, K.; Ling, X.; Marisetty, A.; Ott, M.; Caruso, H.; Barton, E.; Kong, L.Y.; Fang, D.; Latha, K.; et al. Opening of the Blood-Brain Barrier Using Low-Intensity Pulsed Ultrasound Enhances Responses to Immunotherapy in Preclinical Glioma Models. Clin. Cancer Res. 2021, 27, 4325–4337.
More
