Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Alfred Zheng and Version 4 by Alfred Zheng.
Due to the growing demand for eco-friendly products, lithium-ion batteries (LIBs) have gained widespread attention as an energy storage solution. With the global demand for clean and sustainable energy, the social, economic, and environmental significance of LIBs is becoming more widely recognized. LIBs are composed of cathode and anode electrodes, electrolytes, and separators. Notably, the separator, a pivotal and indispensable component in LIBs that primarily consists of a porous membrane material, warrants significant research attention.
- lithium-ion battery
- separator
- membrane
- polymer
- polyethylene
- polypropylene
1. Introduction
Owing to the increasing demand for environmentally-friendly products, LIBs have received widespread attention as an energy storage solution. LIBs are widely considered one of the most sustainable and powerful energy storage technologies available today and are commonly used in industries such as mobile devices, electric vehicles (EVs), and energy storage systems [1][2]. Given the escalating global demand for clean energy and sustainable progress, the social and economic significance of LIBs continues to gain momentum [3][4][5][6]. Due to their remarkable energy density, prolonged storage life, wide operational temperature range, and elevated battery voltage, LIBs have emerged as the predominant contender in the realm of energy storage batteries, finding widespread utility in various domains such as aerospace, artificial satellites, and efficient energy storage for both military and civilian electrical appliances [7]. Additionally, in the current low-carbon global environment, new energy sources have assumed prime importance in the global agenda, specifically with high-capacity LIBs serving as a key power source for 21st-century new-energy EVs [5][8][9]. Notably, as the EV industry and the electronic information sector (encompassing mobile phones, electric tools, and digital cameras) continue to flourish, the demand for LIBs will persistently burgeon [1][10][11]. Therefore, the concept of LIBs has promising prospects, and higher requirements have been put forward for LIB performance, particularly in terms of electrochemical performance (e.g., energy density) and safety [3][12].
LIBs are mainly composed of positive (cathode) and negative (anode) electrodes [13][14], electrolytes, and separators [15][16][17][18][19], wherein the separator, mainly consisting of a porous membrane material, assumes an indispensable and critical role within LIBs (Figure 1). The porous membrane absorbs electrolytes and is assembled between the battery cathode and anode electrodes, which is a crucial section in LIB separators [9][20]. Throughout the charging and discharging cycles of LIBs, lithium ions (Li+) migrate between the cathode and anode electrodes through a separator and, thus, conduct electricity [21]. Specifically, a separator membrane fulfills several vital functions within an LIB. First, it serves to maintain a physical barrier between the cathode and the anode, preventing short circuits arising from direct electrode contact [22]. Second, the presence of micropores in the membrane allows for the passage of Li+, forming a seamless charging and discharging circuit [23]. Furthermore, the membrane exhibits the ability to shut down in response to appropriate temperatures, subsequently closing the micropores, thereby serving as a protective open circuit that safeguards against fires or explosions resulting from thermal runaway caused by elevated temperatures and short circuits. Thus, it offers a protective shield for both battery users and equipment [24][25]. It is worth mentioning that the separator material, typically a thin, polymer-based porous membrane, may be inherently insulating. However, its structural composition and performance characteristics significantly influence the overall performance of LIBs. The basic performance parameters of the membrane mainly encompass thickness, mechanical strength, porosity, wettability, thermal shrinkage, electrochemical stability, and so on [26][27][28]. Therefore, appropriate material selection and structural design of separators determine these performance parameters and play a crucial role in ensuring the safe utilization and long-lasting charging and discharging cycles of LIBs.
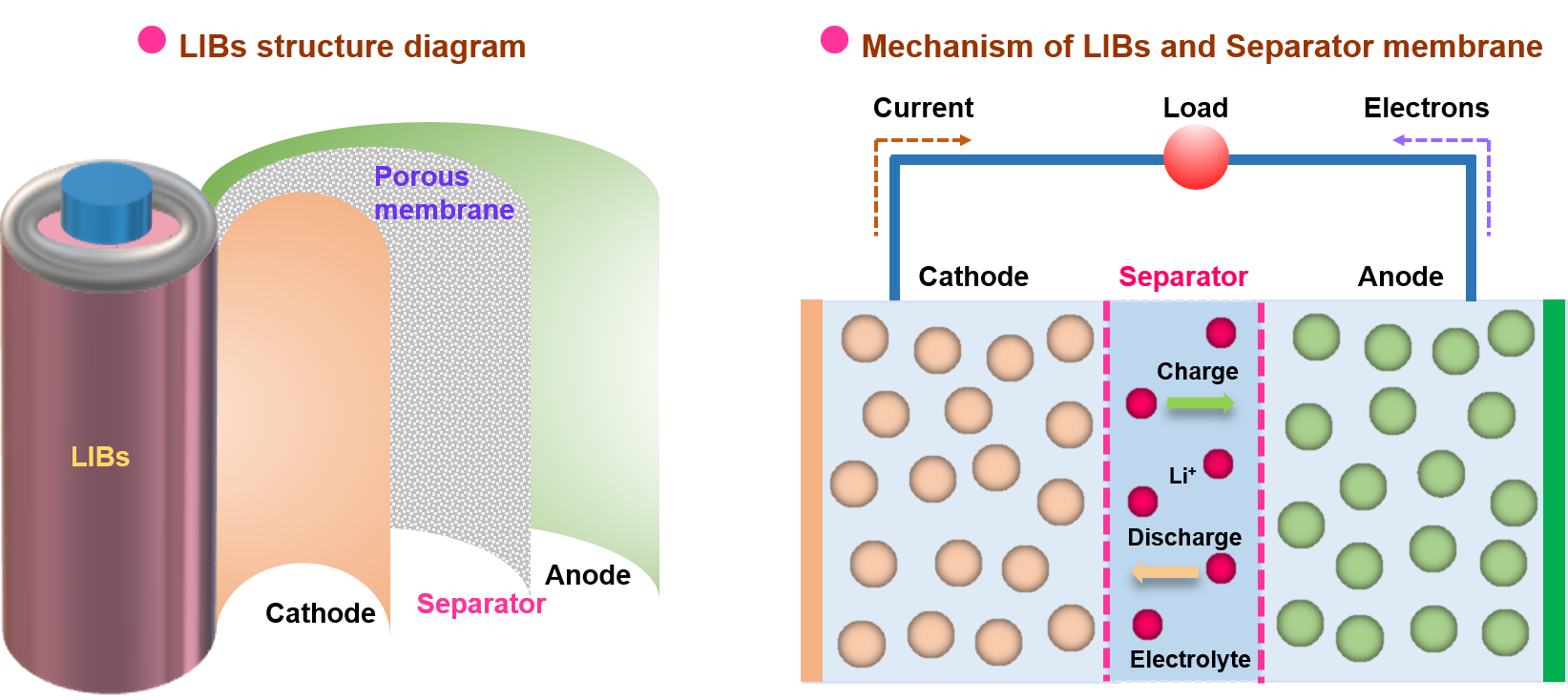
Figure 1. Schematic illustration of rechargeable LIBs consisting of cathode and anode electrodes, electrolytes, and porous membrane-based separators.
Currently, the most commonly utilized polymeric materials for producing porous membranes in rechargeable batteries, particularly LIBs, include polyethylene (PE), polypropylene (PP), poly(tetrafluoroethylene) (PTFE), poly(vinylidene fluoride) (PVDF), poly(methyl methacrylate) (PMMA), polyimide (PI), polyesters, poly(vinyl chloride) (PVC), poly(ethylene oxide) (PEO), polyacrylonitrile (PAN), poly(ethylene terephthalate) (PET), and cellulose and its derivatives [29][30]. Microporous polyolefin membranes, featuring PE, PP, and their blends, hold prominence in the commercial market as separators for secondary rechargeable batteries utilizing liquid electrolytes, including LIB, due to their superior mechanical strength, chemical and electrochemical stability, cost-effective production, tunable pore sizes, thermal shutdown capability, and reasonable material costs [30][31][32]. Notably, ultra-high molecular weight polyethylene (UHMWPE) plays a crucial role in lithium battery separator materials and is highly applied in the global automotive battery market [7][33][34]. Moreover, the UHMWPE membrane provides excellent safety protection for overcharging, short circuit, and explosions when the temperature rises, thus rendering it remarkably suitable for high-efficiency and high-power batteries. At elevated temperatures, the UHMWPE melts smoothly, transitioning into a rubber-like gel without mobility due to the highly entangled molecular chain, thereby impeding undesired membrane collapse [35][36][37]. Preparation methods for polyolefin microporous membranes for LIB separators mainly include the dry method (i.e., melt extrusion stretching method) and the wet method (i.e., thermally induced phase separation method, TIPS) [30][38][39][40][41]. These membranes are created through sequential biaxial stretching, renowned for their remarkable mechanical robustness and consistent pore size distribution [42]. However, these separators manifest noteworthy thermal contraction and exhibit a limited affinity for electrolytes due to their electrolyte-phobic nature and poor electrolyte retention during cycling processes [29][31][43]. These factors compromise their suitability for application in high-power, high-capacity, and high-temperature LIBs. Consequently, over the past decade, numerous researchers have endeavored to address the limitations of conventionally used separators by modifying their structural and material makeup, thereby amplifying the versatility of LIBs across a diverse range of applications, particularly those with elevated power requirements [34][44][45][46].
Notably, the utilization of sustainable electrochemical energy storage devices holds numerous benefits in fostering both a circular and green economy [1][9][10][11][38]. As one of the economically applicable directions for clean energy, the main focus of LIB separators is to develop and prepare new membranes for high-performance batteries [8]. Herein, this resviearchw highlights the significance of porous membrane for separators in LIBs, including the fundamental prerequisites and performance benchmarks of ideal separators, encompassing the chemical, mechanical, electrical, and electrochemical properties of porous separator membranes for batteries. Additionally, this researchview investigates cutting-edge advancements in the preparation, design, modification, and optimization of porous membranes, leveraging polymeric materials including but not limited to PE, UHMWPE, PP, and PVDF. Furthermore, the future prospects of polymer-based composite membranes for LIB applications and the prospective concerns of researchers are outlined. The overall objective of this researchview is to assist relevant researchers in comprehending the underlying design principles, strategies, methodologies, and potential application scenarios associated with porous membranes for LIB separators. Overall, the development of robust, efficient, and innovative LIB separators plays a pivotal role in realizing the tenets of a circular economy, as well as the broader objectives of resource rationalization and environmental sustainability [47].
2. Manufacturing: Dry and Wet Methods Based on Uniaxial/Biaxial Stretching
2.1. Uniaxial/Biaxial Stretching of Separator Membrane
LIB separator membranes can be categorized into several types, encompassing woven or non-woven membranes, microporous membranes, composite membranes, cellulose-based membranes, and electrolyte membranes [34][48][49]. Polyolefin materials such as PE and PP are preferred due to their exceptional mechanical properties, chemical stability, mature manufacturing processes, and low cost; thus, they have been frequently employed as separator components in LIBs research and development [28][34]. While some recent investigations have delved into the utilization of substitute materials (e.g., PVDF) [50][51][52][53], as the primary polymer in phase inversion methods and cellulose-based composite membranes in LIBs [54][55][56][57][58][59][60], commercial polyolefin-based LIB separators still predominated the overall LIBs markets, encompassing PE, PP, and UHMWPE [7][34]. Preparation procedures can be segregated into dry and wet methods, based on the varying formation mechanisms of microporous structures [30][34].
Industrially, the manufacturing focus has been on wet-processed biaxially stretched PE membranes and dry-processed uniaxially stretched PP membranes. Dry stretching can be categorized into uniaxial and biaxial stretching, while wet stretching can be divided into synchronous and asynchronous stretching (Figure 2). Note that dry biaxial stretching relies on the density difference between various phases of PP to create crystal transformation and form microporous membranes. Correspondingly, the wet method, also known as TIPS, utilizes temperature variations to induce phase separation [61]. It is noteworthy that biaxial stretching of PP or PE membranes can be achieved either simultaneously or in sequence. Specifically, sequential biaxial stretching involves stretching the casting membrane in the mechanical direction (MD) while gradually increasing the roller speed. Next, the stretcher frame is stretched in the transverse direction (TD) with the membrane clamped on each side by fasteners connected to the moving chain. The stretched membrane then passes through the heating zone in the oven [62]. Alternatively, simultaneous biaxial stretching can be utilized, wherein the cast or blown membrane is stretched in both MD and TD directions synchronously in a single process, thus preventing scratches and contaminants on the surface of the casting membrane caused by contact with rollers during uniaxial stretching in the MD direction [34].

Figure 2. Schematic illustration of the process flow and corresponding structural evolution of LIB separator membranes based on dry and wet methods.
2.2. Dry Method
The dry method is also known as the melt extrusion stretching method [30]. In a dry process utilizing uniaxial stretching, polyolefin melt is extruded and casted or blown into a membrane, then annealed within the temperature range between glass transition temperature (Tg) and melting temperature (Tm) to increase crystallinity and regulate grain size [63]. Then, the melt is stretched in the MD. Specifically, the blend is melted at elevated temperatures, extruded, and crystallized under the stress field induced by extrusion. Heat treatment is applied subsequently to promote the thickening and refinement of the lamellar crystals [34]. After cold stretching, the lamellar crystals separated into microporous nuclei, followed by hot stretching (to promote microvoid growth) and transformation from lamellar clusters into fibrous crystals. The microporous structure is then fixed by heat-setting to eliminate internal stress. Note that the quality of the prefabricated membrane depends on factors encompassing molecular weight (MW) and distribution (MWD) of the polyolefin resins, as well as extrusion–casting process parameters (i.e., die temperature, tensile ratio, cooling temperature, extrusion rate, etc.) [42]. Moreover, the processing of microporous membranes is influenced by multiple factors including stretching rate and temperature, heat-setting duration and temperature, etc. Overall, the dry stretching method has proven to be a simple and highly efficient technique, yielding a porous membrane that is well-suited for high-power density batteries.
Significantly, despite the dominance of uniaxial stretching technology in the PP membrane market through the dry method, biaxial stretching of PP can be achieved by incorporating β-nucleated isotactic PP (β-iPP) as nucleating agents [64][65]. This process aids in crystal transformation and the formation of microvoids, which occur as a result of differences in density between various phases during stretching. Moreover, this technique optimizes the uniaxial stretching process and balances tensile strength in both MD and TD directions [66]. However, biaxially stretched PP membranes generated from the dry method may have some drawbacks, including excessive thickness, lack of control over pore size and porosity, unevenness, and the need to incorporate pore-forming agents [67][68], which can compromise their practical applications. Notably, a method that takes advantage of the dry method processing has been proposed, wherein the extruded PP membrane is biaxially stretched in both the MD and TD directions without incorporation of β-nucleation agents [34].
2.3. Wet Method
The wet method, also known as the TIPS method, is a technique driven by temperature changes [41][67]. This approach involves melting, swelling, and dissolution of polymers at elevated temperatures to produce a homogeneous solution [34]. In a typical wet method, PE is blended with a solvent of low MW above its Tm, melted to form a homogenous solution, and then extruded into a casting membrane [67]. Notably, paraffin oil is the most commonly used hydrocarbon solvent for PE membranes. After cooling, phase separation occurs, including solid–liquid or liquid–liquid phase separations with a prefabricated membrane formed. It is then heated to a temperature near Tm and then subjected to biaxial stretching in MD and TD to ensure homogeneous molecular chain orientation. Subsequently, the diluent is extracted and recycled, resulting in the formation of a microporous membrane [28]. Note that the wet method yields a microporous membrane featuring comparable tensile strength in both MD and TD directions due to its isotropic properties. However, the anisotropic nature of PP membranes prepared by dry method uniaxial stretching generates a significant disparity in tensile strength between MD and TD, ultimately resulting in poor tear resistance.
Notably, in wet method processes, phase separation is a critical and sophisticated event that involves crystal nucleation, growth, and microcrystalline orientation, occurring after extrusion and cooling. Moreover, nucleation, growth, destruction, and reconstruction of crystals, as well as the nonlinear evolution of multi-scale structures such as lamellar crystals, amorphous regions, fibrous crystals, and microvoids occur through synchronous or asynchronous biaxial stretching [30]. Subsequently, the solvent is extracted with a suitable extractant with a low boiling point, and internal stresses are then alleviated by heat treatment, leading to the development of a cohesive microporous structure (Figure 2). Therefore, the preparation of microporous membranes is a multifaceted coupling outcome of several phases and multiple courses involving various processing parameters, dependent on multifarious factors encompassing MW, MWD, material concentration, and processing conditions including extrusion molding, casting, biaxial stretching, heat-setting, and so on [62]. Note that the predominant advantage of PE separator membranes lies in their superior mechanical strength, which is well balanced in both directions. This can be attributed to their less anisotropic pore structure, which distinguishes them from uniaxially stretched PP-based membranes [28][30].
References
- Di Lecce, D.; Verrelli, R.; Hassoun, J. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467.
- Chae, B.-G.; Park, S.Y.; Song, J.H.; Lee, E.; Jeon, W.S. Evolution and expansion of Li concentration gradient during charge–discharge cycling. Nat. Commun. 2021, 12, 3814.
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884.
- Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 2001, 1, 406–413.
- Lin, L.; Ning, H.; Song, S.; Xu, C.; Hu, N. Flexible electrochemical energy storage: The role of composite materials. Compos. Sci. Technol. 2020, 192, 108102.
- Babiker, D.M.D.; Usha, Z.R.; Wan, C.; Hassaan, M.M.E.; Chen, X.; Li, L. Recent progress of composite polyethylene separators for lithium/sodium batteries. J. Power Sources 2023, 564, 232853.
- McCrossan, C.; Shankaravelu, K. A review of the second life electric vehicle battery landscape from a business and technology perspective. In Proceedings of the 2021 IEEE Green Technologies Conference (GreenTech), Denver, CO, USA, 7–9 April 2021; pp. 416–423.
- Manjakkal, L.; Jain, A.; Nandy, S.; Goswami, S.; Tiago Carvalho, J.; Pereira, L.; See, C.H.; Pillai, S.C.; Hogg, R.A. Sustainable electrochemical energy storage devices using natural bast fibres. Chem. Eng. J. 2023, 465, 142845.
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332.
- Weidenkaff, A.; Wagner-Wenz, R.; Veziridis, A. A world without electronic waste. Nat. Rev. Mater. 2021, 6, 462–463.
- Qi, Z.; Wang, H. Advanced thin film cathodes for lithium ion batteries. Research 2020, 2020, 2969510.
- Yang, Y.; Chen, Z.; Lv, T.; Dong, K.; Liu, Y.; Qi, Y.; Cao, S.; Chen, T. Ultrafast self-assembly of supramolecular hydrogels toward novel flame-retardant separator for safe lithium ion battery. J. Colloid Interface Sci. 2023, 649, 591–600.
- Liu, F.; Chuan, X. Recent developments in natural mineral-based separators for lithium-ion batteries. RSC Adv. 2021, 11, 16633–16644.
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533.
- Zhang, J.; Zhang, H.; Weng, S.; Li, R.; Lu, D.; Deng, T.; Zhang, S.; Lv, L.; Qi, J.; Xiao, X.; et al. Multifunctional solvent molecule design enables high-voltage Li-ion batteries. Nat. Commun. 2023, 14, 2211.
- Ryu, M.; Hong, Y.-K.; Lee, S.-Y.; Park, J.H. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 2023, 14, 1316.
- Zhu, N.; Zhang, K.; Wu, F.; Bai, Y.; Wu, C. Ionic liquid-based electrolytes for aluminum/magnesium/sodium-ion batteries. Energy Mater. Adv. 2021, 2021, 9204217.
- Dong, W.; Lin, T.; Huang, J.; Wang, Y.; Zhang, Z.; Wang, X.; Yuan, X.; Lin, J.; Chen, I.-W.; Huang, F. Electrodes with electrodeposited water-excluding polymer coating enable high-voltage aqueous supercapacitors. Research 2020, 2020, 4178179.
- Langner, T.; Sieber, T.; Rietig, A.; Merk, V.; Pfeifer, L.; Acker, J. A phenomenological and quantitative view on the degradation of positive electrodes from spent lithium-ion batteries in humid atmosphere. Sci. Rep. 2023, 13, 5671.
- Tajik, M.; Makui, A.; Tosarkani, B.M. Sustainable cathode material selection in lithium-ion batteries using a novel hybrid multi-criteria decision-making. J. Energy Storage 2023, 66, 107089.
- Hao, H.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.; Mitlin, D. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal–sulfur and selenium batteries. Chem. Rev. 2022, 122, 8053–8125.
- Gu, Q.-Q.; Xue, H.-J.; Li, Z.-W.; Song, J.-C.; Sun, Z.-Y. High-performance polyethylene separators for lithium-ion batteries modified by phenolic resin. J. Power Sources 2021, 483, 229155.
- Li, Y.; Yu, L.; Hu, W.; Hu, X. Thermotolerant separators for safe lithium-ion batteries under extreme conditions. J. Mater. Chem. A 2020, 8, 20294–20317.
- Lin, W.; Wang, F.; Wang, H.; Li, H.; Fan, Y.; Chan, D.; Chen, S.; Tang, Y.; Zhang, Y. Thermal-stable separators: Design principles and strategies towards safe lithium-ion battery operations. ChemSusChem 2022, 15, e202201464.
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Application of organic-inorganic hybrids in lithium batteries. Mater. Today Phys. 2020, 15, 100289.
- Wu, Y.; Lei, D.; Wang, C. The formation of LiAl5O8 nanowires from bulk Li-Al alloy enables dendrite-free Li metal batteries. Mater. Today Phys. 2021, 18, 100395.
- Luo, W.; Cheng, S.; Wu, M.; Zhang, X.; Yang, D.; Rui, X. A review of advanced separators for rechargeable batteries. J. Power Sources 2021, 509, 230372.
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent development in separators for high-temperature lithium-ion batteries. Small 2019, 15, 1901689.
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886.
- Liu, R.; Yuan, B.; Zhong, S.; Liu, J.; Dong, L.; Ji, Y.; Dong, Y.; Yang, C.; He, W. Poly(vinylidene fluoride) separators for next-generation lithium based batteries. Nano Select 2021, 2, 2308–2345.
- Zhai, P.; Liu, K.; Wang, Z.; Shi, L.; Yuan, S. Multifunctional separators for high-performance lithium ion batteries. J. Power Sources 2021, 499, 229973.
- Yoneda, H.; Nishimura, Y.; Doi, Y.; Fukuda, M.; Kohno, M. Development of microporous PE films to improve lithium ion batteries. Polym. J. 2010, 42, 425–437.
- Mun, S.C.; Won, J.H. Manufacturing processes of microporous polyolefin separators for lithium-ion batteries and correlations between mechanical and physical properties. Crystals 2021, 11, 1013.
- Deplancke, T.; Lame, O.; Rousset, F.; Seguela, R.; Vigier, G. Mechanisms of chain reentanglement during the sintering of UHMWPE nascent powder: Effect of molecular weight. Macromolecules 2015, 48, 5328–5338.
- Zhao, Y.; Liang, Y.; Yao, Y.; Wang, H.; Lin, T.; Gao, Y.; Wang, X.; Xue, G. Chain dynamics of partially disentangled UHMWPE around melting point characterized by 1H low-field solid-state NMR. Polymers 2023, 15, 1910.
- Shen, H.; He, L.; Fan, C.; Xie, B.; Yang, W.; Yang, M. Improving the integration of HDPE/UHMWPE blends by high temperature melting and subsequent shear. Mater. Lett. 2015, 138, 247–250.
- Wang, W.; Zhang, Z.; Ma, L.; Xu, X.; Zhang, P.; Yu, H. Explorations of complex thermally induced phase separation (C-TIPS) method for manufacturing novel diphenyl ether polysulfate flat microporous membranes. J. Membr. Sci. 2022, 659, 120739.
- Cheng, Q.; Cui, Z.; Li, J.; Qin, S.; Yan, F.; Li, J. Preparation and performance of polymer electrolyte based on poly(vinylidene fluoride)/polysulfone blend membrane via thermally induced phase separation process for lithium ion battery. J. Power Sources 2014, 266, 401–413.
- Kim, J.F.; Kim, J.H.; Lee, Y.M.; Drioli, E. Thermally induced phase separation and electrospinning methods for emerging membrane applications: A review. AIChE J. 2016, 62, 461–490.
- Lee, J.T.; Jo, C.; De Volder, M. Bicontinuous phase separation of lithium-ion battery electrodes for ultrahigh areal loading. Proc. Natl. Acad. Sci. USA 2020, 117, 21155–21161.
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462.
- Choi, Y.; Kim, J.I.; Moon, J.; Jeong, J.; Park, J.H. Electron beam induced strong organic/inorganic grafting for thermally stable lithium-ion battery separators. Appl. Surf. Sci. 2018, 444, 339–344.
- Zhao, J.; Fan, R.; Xiang, S.; Hu, J.; Zheng, X. Preparation and lithium-ion separation property of ZIF-8 membrane with excellent flexibility. Membranes 2023, 13, 500.
- Shkrob, I.A.; Luo, M.; Rodrigues, M.-T.F.; Trask, S.E.; Abraham, D.P. Fast charging of Li-ion cells: Effect of separator membranes and mapping of “safe lines” to avoid Li plating. J. Power Sources 2022, 549, 232086.
- Heidari, A.A.; Mahdavi, H. Recent development of polyolefin-based microporous separators for Li−ion batteries: A review. Chem. Rec. 2020, 20, 570–595.
- Serra, J.P.; Fidalgo-Marijuan, A.; Martins, P.M.; Queirós, J.M.; Gonçalves, R.; Gutiérrez-Pardo, A.; Aguesse, F.; Costa, C.M.; Lanceros-Mendez, S. Porous composite bifunctional membranes for lithium-ion battery separator and photocatalytic degradation applications: Toward multifunctionality for circular economy. Adv. Energy Sustain. Res. 2021, 2, 2100046.
- Jeon, D.H. Wettability in electrodes and its impact on the performance of lithium-ion batteries. Energy Storage Mater. 2019, 18, 139–147.
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375.
- Sohn, J.-Y.; Im, J.S.; Gwon, S.-J.; Choi, J.-H.; Shin, J.; Nho, Y.-C. Preparation and characterization of a PVDF-HFP/PEGDMA-coated PE separator for lithium-ion polymer battery by electron beam irradiation. Radiat. Phys. Chem. 2009, 78, 505–508.
- Wang, J.; Shen, J.; Shi, J.; Li, Y.; You, J.; Bian, F. Crystallization-templated high-performance PVDF separator used in lithium-ion batteries. J. Membr. Sci. 2023, 670, 121359.
- Folayan, T.-O.; Zhan, R.; Huang, K.; Pan, L. Improved separation between recycled anode and cathode materials from Li-ion batteries using coarse flake particle flotation. ACS Sustain. Chem. Eng. 2023, 11, 2917–2926.
- Zhao, Y.; Fang, L.-Z.; Kang, Y.-Q.; Wang, L.; Zhou, Y.-N.; Liu, X.-Y.; Li, T.; Li, Y.-X.; Liang, Z.; Zhang, Z.-X.; et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Metals 2021, 40, 1431–1436.
- Zhang, J.; Chen, S.; Xie, X.; Kretschmer, K.; Huang, X.; Sun, B.; Wang, G. Porous poly(vinylidene fluoride-co-hexafluoropropylene) polymer membrane with sandwich-like architecture for highly safe lithium ion batteries. J. Membr. Sci. 2014, 472, 133–140.
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. TiO2 ceramic-grafted polyethylene separators for enhanced thermostability and electrochemical performance of lithium-ion batteries. J. Membr. Sci. 2016, 504, 97–103.
- Nunes-Pereira, J.; Kundu, M.; Gören, A.; Silva, M.M.; Costa, C.M.; Liu, L.; Lanceros-Méndez, S. Optimization of filler type within poly(vinylidene fluoride-co-trifluoroethylene) composite separator membranes for improved lithium-ion battery performance. Compos. Part B Eng. 2016, 96, 94–102.
- Tan, Z.; Wang, X.; Fu, C.; Chen, C.; Ran, X. Effect of electron beam irradiation on structural and thermal properties of gamma poly (vinylidene fluoride) (γ-PVDF) films. Radiat. Phys. Chem. 2018, 144, 48–55.
- Valentino, L.; Matsumoto, M.; Dichtel, W.R.; Mariñas, B.J. Development and performance characterization of a polyimine covalent organic framework thin-film composite nanofiltration membrane. Environ. Sci. Technol. 2017, 51, 14352–14359.
- Feng, J.; Zhang, G.; MacInnis, K.; Olah, A.; Baer, E. Structure-property relationships of microporous membranes produced by biaxial orientation of compatibilized PP/Nylon 6 blends. Polymer 2018, 145, 148–156.
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001.
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468.
- Zhong, S.; Yuan, B.; Guang, Z.; Chen, D.; Li, Q.; Dong, L.; Ji, Y.; Dong, Y.; Han, J.; He, W. Recent progress in thin separators for upgraded lithium ion batteries. Energy Storage Mater. 2021, 41, 805–841.
- Ding, L.; Yan, N.; Zhang, S.; Xu, R.; Wu, T.; Yang, F.; Cao, Y.; Xiang, M. Facile manufacture technique for lithium-ion batteries composite separator via online construction of fumed SiO2 coating. Mater. Des. 2022, 215, 110476.
- Wang, C.; Zhang, Z.; Ding, Q.; Jiang, J.; Li, G.; Mai, K. β-Crystallization of isotactic polypropylene in the presence of β-nucleating agent and different crystallinity poly(ethylene terephthalate). Thermochim. Acta 2013, 559, 17–22.
- Zhang, D.-X.; Ding, L.; Yang, F.; Lan, F.; Cao, Y.; Xiang, M. Comparison of the structural evolution of β polypropylene during the sequential and simultaneous biaxial stretching process. Chin. J. Polym. Sci. 2021, 39, 620–631.
- Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. β-Nucleated polypropylene: Processing, properties and nanocomposites. Polym. Rev. 2015, 55, 596–629.
- Wu, Y.; Yang, F.; Cao, Y.; Xiang, M.; Kang, J.; Wu, T.; Fu, Q. Investigation on cavitation behavior of ultrahigh molecular weight polyethylene during stretching in wet process and dry process. Polymer 2021, 230, 124081.
- Carmeli, E.; Ottonello, S.; Wang, B.; Menyhárd, A.; Müller, A.J.; Cavallo, D. Competing crystallization of α- and β-phase induced by β-nucleating agents in microdroplets of isotactic polypropylene. CrystEngComm 2022, 24, 1966–1978.
More
