Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Sonil Nanda.
Biofuels are the sustainable counterparts of fossil fuels to meet the increasing energy demands of the current and future generations. Biofuels are produced from waste organic residues with the application of mechanical, thermochemical and biological methods and processes. While mechanical and thermochemical conversion processes involve the use of heat, pressure, catalysts and other physicochemical attributes for the direct conversion of biomass, biological conversion requires microorganisms and their enzymes as biocatalysts to degrade the fermentable substrates into biofuels and biochemicals.
- biobutanol
- bioconversion
- biodiesel
- bioethanol
- biofuels
1. Bioethanol
Bioethanol is a liquid biofuel constituted of a significantly higher oxygen content of 35%, which can decrease vehicular emissions. Bioethanol is often blended with gasoline at flexible propositions for use as a drop-in biofuel in existing motor engines [45][1]. The use of bioethanol in the replacement of conventional fuels could boost the performance and economy of vehicular engines due to its low carbon footprint. Meanwhile, due to its poor volumetric energy density compared to regular gasoline, the vehicles need more volume of bioethanol, unlike conventional fuels. To overcome this problem, bioethanol can be used as a blending component in conventional liquid fuel. Bioethanol is produced from various lignocellulosic and sugar-based feedstocks like agricultural residues (e.g., straw, bran and stalk), food-processing wastes and different energy crops using pretreatment, enzymatic hydrolysis and fermentation.
Baker’s yeast (Saccharomyces cerevisiae) is a model microorganism that can ferment starch and hexose sugars (i.e., glucose) to produce bioethanol through the simple glycolytic pathway. It should be noted that first-generation biomasses, such as corn, potato and cassava, are rich in starch and/or glucose, which are the preferred feedstocks for biorefineries for fermentation to produce bioethanol because the diversion of these food crops for fuel products instead of consumption led to food-versus-fuel criticism [46][2]. On the contrary, second-generation or lignocellulosic biomass, such as agricultural and wood-based residues, have emerged as alternative feedstocks for bioethanol and other biofuel production [47][3]. However, lignocellulosic biomass poses several upstream challenges for fermentation due to the presence of lignin, which hinders the access of enzymes and microorganisms to directly biodegrade holocellulose (cellulose and hemicellulose) sugars. Although the pretreatment of biomass could separate lignin from holocellulose, it may result in the generation of certain degradation products, such as carboxylic acids, phenols and furfurals, which reduce the pH of the hydrolysate and also inhibit the growth and activity of fermenting microorganisms [48][4]. In addition, S. cerevisiae lacks the natural metabolic pathway to ferment pentose sugars (monomers of hemicellulose), thus impacting the fermentation of lignocellulosic biomass to bioethanol [49][5]. Nonetheless, continued research and development in biomass pretreatment processes, bioprocess engineering and genetic engineering of microorganisms have led to significant progress in the fermentation of lignocellulosic materials to bioethanol. One such approach is the use of thermophilic bacteria for bioethanol production. Thermophilic bacteria are extremophilic microorganisms that can proliferate under extreme temperatures, pressure, osmosis, salinity and radiation conditions [50][6]. Thermophilic microorganisms harbor unique enzymes that make them thermally and physiologically robust, with an array of advantages for bioprocessing, such as stability against contamination and inhibitors, enhanced reaction kinetics, improved product yields and accelerated organic matter degradation [51,52,53][7][8][9].
Figure 1 represents the typical glycolytic fermentation pathway to produce ethanol using glucose as a simple sugar. S. cerevisiae deploys the Embden–Meyerhoff–Parnas glycolytic pathway, whereas Zymomonas mobilis uses the Entner–Doudoroff pathway for glucose metabolism [54][10]. The Entner–Doudoroff pathway is mainly an aerobic route for glucose metabolism and is widely found in Pseudomonas spp. The Entner–Doudoroff pathway theoretically yields 2 moles of adenosine triphosphate (ATP) per mole of glucose fermented to ethanol. On the other hand, the Entner–Doudoroff pathway releases 1 mol/mol of ATP, which also results in low cell mass and allows higher ethanol yields [54][10].
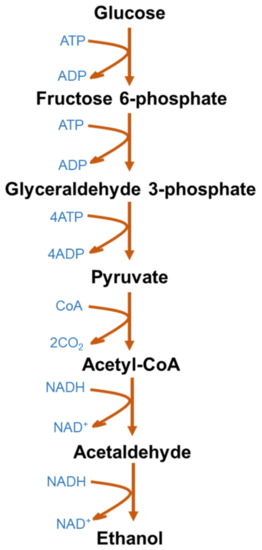
Figure 1.
Microbial metabolic pathway for bioethanol production from glucose.
There have been several studies on the fermentation of sugars to produce bioethanol in the last few decades. In a study by Raud et al. [55][11], barley straw was used to produce bioethanol using a three-step process, which included liquid hot water pretreatment at 125–175 °C with an external N2 pressure of 3 MPa, followed by hydrolysis and fermentation. The hydrolysis of pretreated feedstock was performed using a commercial biocatalyst or enzyme (Accellerase 1500) at 50 °C for 72 h. The enzymatic hydrolysis was followed by fermentation using the conventional fungi (S. cerevisiae) at 22 °C for 7 days, which resulted in a bioethanol yield of 0.43–0.9 g/g. The higher pretreatment temperature resulted in enhanced degradation of non-cellulosic moieties, which subsequently increased the availability of cellulose for enzymatic hydrolysis and fermentation. Barley straw required pretreatment for the decomposition of non-cellulosic components and loosening of the strong intermolecular or intramolecular linkages in the lignocellulosic matrix for the enhanced recovery of fermentable sugars. However, food processing wastes comprised of fermentable sugars that could be directly fermented into bioethanol without any intensive pretreatment.
Khoshkho et al. [61][12] used waste carrot pulp for fermentation using S. cerevisiae at 28 °C in 72 h to produce a bioethanol concentration and yield of 40.6 g/L and 0.57 g/g, respectively. The enhanced production of bioethanol was attributed to the use of beet molasses during the fermentation process, which significantly activated the microbial community due to the presence of pre-fed sugars through molasses. In addition to the two-step fermentation process, consolidated bioprocessing has become more economically feasible for bioethanol fermentation using genetically engineered thermophilic bacteria, such as Thermoanaerobacterium saccharolyticum [62][13].
In a study by Qu et al. [59][14], soybean straw and sorghum stalk were used as feedstocks for fermentation after mild acid pretreatment. The fermentation process was performed in an anaerobic environment with an engineered thermophilic bacterium Thermoanaerobacterium aotearoense SCUT27/ΔargR1864 at 55 °C, which provided the highest bioethanol yield of 0.34 and 0.36 g/g from the soybean straw and sorghum stalk, respectively.
Raita et al. [57][15] used de-oiled palm kernel cake for a three-step conversion into bioethanol. The de-oiled cake was pretreated using a steam explosion technique followed by enzymatic hydrolysis using a bi-enzyme system containing SEB mannanase and CTec2 cellulase at 50 °C for 72 h. The fermentation of hydrolyzed feedstock was performed using a thermophilic bacterium strain Geobacillus thermoglucosidasius TM242, which produced a significantly high yield of bioethanol (0.47 g/g). Unlike mesophilic microbial systems, thermophilic anaerobic bacteria have the advantage of higher bioethanol yield due to the significant reduction of the oxidation reaction and utilization of a wide range of hexose and pentose sugars [63][16].
Sivarathnakumar et al. [56][17] utilized mesquite stem as a lignocellulosic feedstock to produce bioethanol, where the biomass was treated with mild nitric acid before saccharification and fermentation processes. The simultaneous saccharification and fermentation process was performed in a single-compartment fermenter in the presence of the commercially available cellulase (activity of 12 FPU/g of biomass) and Kluyveromyces marxianus MTCC 1389 as the fermentative bacteria. The entire saccharification and fermentation were performed at 41 °C and pH 4.9 in 72 h to obtain a bioethanol concentration and yield of 21.5 g/L and 0.67 g/g, respectively.
Tse et al. [64][18] investigated bioethanol production from various feedstocks and reported that barley straw had a maximum bioethanol yield of 1.14 L/kg compared to that of corn stover, sugarcane bagasse and wheat straw, which revealed a bioethanol yield in the range of 0.4–0.6 L/kg. A bioethanol yield of 1.5 L/kg has also been reported from brown macroalgae [64][18].
2. Biobutanol
Butanol is categorized into four active isomers, 1-butanol, 2-butanol, iso-butanol and tert-butanol. The physicochemical properties of biobutanol suggest it is a superior fuel additive due to its higher calorific value of 29 MJ/L as compared to bioethanol (19.6 MJ/L) [65][19]. Compared to bioethanol, biobutanol has lower volatility, higher miscibility in gasoline and considerably fewer issues during ignition in vehicle engines [18][20]. Due to these superior physicochemical properties, biobutanol can be used as an additive in various fossil fuels, like gasoline and diesel, or as a drop-in biofuel without any modification of vehicular engines. In addition to fuel applications, butanol has several applications as a routine laboratory solvent in the chemical, polymer, textiles, paints and cosmetic industries. Biobutanol is biologically produced using one of the oldest fermentation processes, namely acetone–butanol–ethanol (ABE) fermentation using different species of the anaerobic bacteria Clostridium.
The metabolic pathway of ABE fermentation is presented in Figure 2. The production of butanol is regulated by several enzymes, namely acetyl-CoA, acetoacetyl-CoA, 3-hydroxybutyryl-CoA, crotonyl-CoA and butyryl CoA [66][21]. Clostridium metabolizes glucose to produce pyruvate, which is converted into acetyl-CoA by the tricarboxylic acid cycle. Acetyl-CoA is further converted into acetic acid and butyryl-CoA via acetoacetyl-CoA and 3-hydroxybutyryl-CoA. Butyryl-CoA is metabolized to butyric acid, butyraldehyde and butanol. Acetic acid, butyric acid, acetone, ethanol, H2 and CO2 are also obtained as byproducts of ABE fermentation along with butanol. The typical yield ratio of acetone, butanol and ethanol from ABE fermentation orchestrated by Clostridium spp. is 3:6:1 [67][22]. Recent research efforts have reported developing efficient bioprocesses and microorganisms that can co-utilize the byproducts, such as lactic acid and acetic acid, to produce biobutanol [68][23].
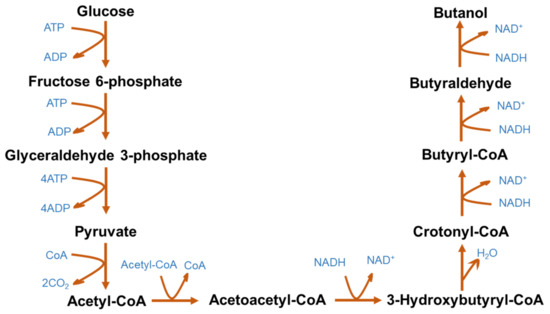
Figure 2.
Microbial metabolic pathway for acetone–butanol–ethanol fermentation.
Wen et al. [71][24] used two clostridial strains, Clostridium cellulovorans DSM 743B and Clostridium beijerinckii NCIMB 8052, for the conversion of corn cob into biobutanol. Before the fermentation process, the feedstock was treated with a mild alkali solution for the removal of lignin and loosening of the cellulosic bond structures. Moreover, C. cellulovorans was genetically engineered by removing the acetate and lactic acid-forming genes, acetate kinase and lactate dehydrogenase, respectively. This subsequently overexpressed the butyryl-forming genes to produce butyryl kinase. For C. beijerinckii, the genes responsible for biobutanol formation through organic acid reassimilation and metabolism of pentose sugars, ctfAB, cbei_3833/3834, xylR, cbei_2385, xylT and cbei_0109, were also overexpressed for the enhanced production of biobutanol. The overexpression of these genes diverts the metabolic pathway of the bacteria toward the butyryl formation and subsequently increases the biobutanol yield.
Tsai et al. [75][25] used rice straw as the feedstock to produce biobutanol through a separate hydrolysis and fermentation route. In this preliminary step, the feedstock was pretreated using a mild solution of hydrogen peroxide for better digestion of the feedstock due to the degradation of the lignin and weakening of different cellulosic and hemicellulosic linkages. The pretreated rice straw was hydrolyzed using the Accellerase 1500 enzyme followed by ABE fermentation using Clostridium acetobutylicum ATCC 824, which produced 23 wt/wt% biobutanol. This showed a comparatively higher biobutanol yield than the other studies described above, which was attributed to the immobilization of the clostridial stain on polyvinyl alcohol. The immobilization of C. acetobutylicum enhanced cell loading into the fermenter, which decreased the lag phase, increasing the sugar conversion rate and biobutanol yield. Thus, the immobilization of enzymes and microorganisms is considered another technique for the enhancement of biobutanol or other alcohol fermentation processes.
3. Biomethane
Anaerobic digestion is well established as a traditional technique for the bioconversion of organic waste and sludge into biogas or biomethane. This technique is promising for degrading solid residues from sewage treatment plants and fermentation processes to simultaneously produce biomethane while valorizing the wastes [79,80][26][27]. As shown in Figure 3, the anaerobic digestion process is constituted of four major steps, including hydrolysis, acidogenesis, acetogenesis and methanogenesis [81][28]. Biomethane has the potential to replace natural gas for both stationary and mobile applications due to its higher calorific value of around 36 MJ/m3.
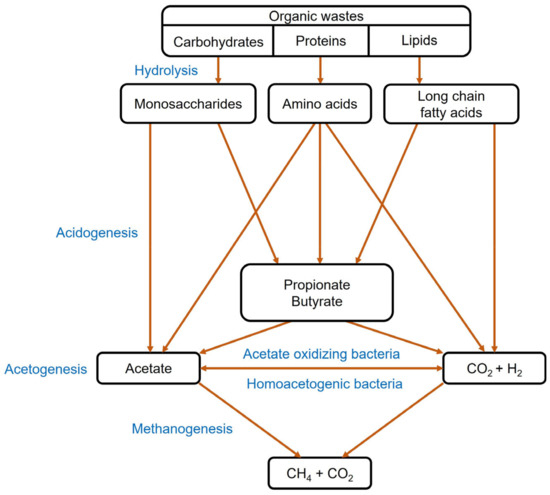
Figure 3.
Conversion of organic waste into biomethane through anaerobic digestion.
In addition to anaerobic digestion, the co-digestion of different organic wastes provides a large corridor for industrial research due to its advantages of the synergistic effects of the substrates to maintain the pH and carbon/nitrogen ratio, which plays a vital role in biomethane production. Anaerobic digestion occurs in the absence of oxygen with the application of common methanogenic bacteria under the genera Methanobrevibacter, Methanococcus, Methanogenium, Methanopyrus, Methanosaeta, Methanosarcina and Methanosphaera.
Syntrophic metabolism mediated through interspecies electron transfer plays a significant role in anaerobic digestion, especially in the oxidation of volatile fatty acids [82][29]. In this mechanism, the redox mediator generated by the biological oxidation of volatile fatty acids is transferred between methanogenic and non-methanogenic bacteria via interspecies electron transfer. This interspecies hydrogen transfer can decrease the partial pressure of hydrogen to less than 10−4 atm, facilitating the emergence of acetogenic reactions [83][30].
Latifi et al. [91][31] utilized the organic waste generated from the slaughterhouse (e.g., blood, meat pieces and feathers) along with sewage sludge for biomethane production through anaerobic co-digestion. The authors optimized the effects of the total solid content of the feedstock (e.g., 5 and 7 wt%) and the inoculum/substrate ratio (e.g., 1, 2 and 4) on biomethane yield. It was observed that at a total solid content of 5 wt% and inoculum/substrate ratio of 4, the co-digestion of the feedstocks produced the highest biogas yield of 631 mL/g volatile solids (VS), with a biomethane fraction of 73% under mesophilic conditions using secondary sludge as the inoculum, which can be considered one of the highest biomethane fraction obtained in biogas. The lower solid-constituted substrate provided a higher biogas yield due to the lower accumulation of volatile fatty acids in the reactor, which lessened the negative effects due to the volatile fatty acid accumulation.
Elsayed et al. [86][32] studied the effects of inoculum on biomethane generation from the co-digestion of fruit–vegetable waste (FVW) and the primary sludge (PS) using activated sludge (or secondary sludge) as the inoculum. This delivered the highest biomethane yields of 141 mL/g VS and 295 mL/g VS without and with inoculum, respectively. The optimized parameters, including the temperature, retention time, and the primary sludge-to-FVW and inoculum/substrate ratios were determined as 37 °C, 30 days, 50:50 and 2, respectively. It was observed that feedstock with a higher component of fruits and vegetable waste (PS/FVW ratio of 20:80) delivered the lowest biomethane yield due to the accumulation of volatile fatty acids, which affected the growth and activity of methanogenic bacteria and subsequently decreased the biomethane yield.
The mixing of different substrates in the proper ratio governs the pH and carbon/nitrogen ratio of the medium, which prominently affects the yield of biomethane. In addition to the production of biomethane, anaerobic digestion has become a potential component in wastewater treatment processes. It was employed to remove organic solids from various wastewater and sludge generated from different process industries and municipal solid or liquid wastes [87,90][33][34].
4. Biohydrogen
Hydrogen is a clean and versatile energy vector and carrier. Hydrogen energy has long been considered energy for the future [92][35]. The sustainable production of hydrogen from renewable resources plays an important role in the global energy transition. The hydrogen economy is also expected to increase with the implementation of the existing renewable energy strategies [93][36]. However, the industrial-scale production of hydrogen is still heavily dependent on fossil energy resources through the steam reforming of methane (i.e., natural gas), which is considered not sustainable due to significant carbon emissions [94,95][37][38]. Therefore, finding alternatives to produce hydrogen in a renewable manner and with a cleaner process has been a hot research topic for decades.
Currently, there are many different hydrogen production routes mainly categorized into thermochemical (e.g., gasification and pyrolysis), electrochemical (e.g., electrolysis), photocatalytic and biological (e.g., dark fermentation and photo-fermentation) [96][39]. In the photo and dark fermentation processes, organic matter (e.g., sugars derived from different sources, food waste, agricultural residues, sewage sludge and wastewater) is used as a substrate for biodegradation by microorganisms [97][40]. The advantages of dark fermentation include (i) the production of biohydrogen without a light source, (ii) a higher bioproduction rate compared to biophotolysis and photo fermentation, (iii) flexibility in using diverse and low-cost feedstocks and (iv) adaptability to perform the fermentation process using existing bioreactor designs [98][41]. During the photo and dark fermentation processes, several critical factors that significantly impact biohydrogen yield and selectivity include the physicochemical properties and loading of the feedstocks, the type of fermenting microorganism, the process parameters, including the temperature, reaction media, time, agitation, aerobic or anaerobic conditions, bioreactor design and feeding mode (i.e., batch, fed-batch, or continuous) and catabolic enzymes [99,100][42][43].
The partial pressure of hydrogen is also a critical factor of dark fermentation. Fermentative biohydrogen production is negatively impacted at a higher partial pressure of hydrogen, which requires the intermittent degassing of the enclosed bioreactors with inert gases, such as N2, to enhance the liquid-to-gas mass transfer [101][44]. According to Henry’s Law, the liquid concentration and the partial pressure of hydrogen are theoretically linked at thermodynamic equilibrium [102][45].
Like ABE fermentation, several Clostridium spp. are also responsible for performing dark fermentation to produce biohydrogen through the metabolism based on NADH-for and pyruvate ferredoxin oxidoreductase (pfor) [103][46]. The reduced ferredoxin (Fd) and NADH lead to the reduction of H+ ions catalyzed by hydrogenase (hyd), thus resulting in biohydrogen production via dark fermentation. As shown in Figure 4, NADH produced from glycolysis is oxidized, resulting in the release of 2 mol of H2 by the reduction of H+ ions. On the other hand, ferredoxin is reduced via the oxidation of pyruvate to acetyl coenzyme A (CoA), leading to biohydrogen production. Two moles of biohydrogen are also produced by the oxidation of the reduced ferredoxin [104][47]. Hence, totals of 2 moles and 4 moles of H2 are produced when butyrate and acetate are the final products, respectively. Similarly, a hyperthermophilic anaerobic bacterium, Thermotoga maritima, deploys its iron hydrogenase enzyme to synergistically utilize NADH and ferredoxin to produce biohydrogen [105][48]. In addition, the anaerobic bacterium Syntrophomonas wolfei hosting a multimeric [FeFe]-hydrogenase enzyme demonstrates biohydrogen production when co-cultured with hydrogen- and/or formate-using methanogen via fatty-acid oxidization [106][49]. [FeFe]-hydrogenase has been reported to be ferredoxin-independent and NADH-dependent to re-oxidize NADH.
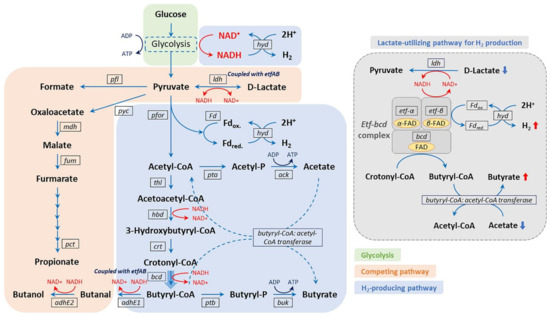
Figure 4. Microbial metabolic pathway for biohydrogen production. Reproduced with permission from Kim et al. [103].
Biohydrogen production from lignocellulosic substrates can be facilitated under thermophilic conditions. Hyperthermophilic bacteria exhibit a theoretical biohydrogen production potential of 4 mol/mol via dark fermentation [107][50]. Thermophilic bacteria, such as Caldicellulosiruptor saccharolyticus, Thermotoga neapolitana and Thermotoga maritima, demonstrate a wide variety of physiological and metabolic properties to aid dark fermentation and improve biohydrogen production [108,109,110][51][52][53]. Some of these physiological properties distinctive to (hyper)thermophilic bacteria include hydrolytic capability (via glycoside hydrolase), substrate degradation, tolerance to inhibition and stress conditions, thermal stability and enhanced regulation of redox and carbon metabolism pathways [111][54].
Chen et al. [97][40] studied the effects of the temperature of the content of total solids on biohydrogen production via dark fermentation of rice straw operated under thermophilic (55 °C) and mesophilic (37 °C) conditions. The results suggest that the butyric acid fermentation pathway was the primary biohydrogen production route for both the thermophilic and mesophilic dark fermentation processes. The thermophilic dark fermentation process showed a higher biohydrogen yield than that operated under mesophilic conditions. The doubling in total solids content from 6% to 12% resulted in a shift of the conversion pathway, leading to better improvement in the biomethane generation compared to biohydrogen.
Li et al. [112][55] studied biohydrogen generation from the dark fermentation of activated sludge. The results reveal that the addition of rhamnolipid, an environmentally friendly biosurfactant, improved the biohydrogen yield. Another recent trend to enhance biohydrogen production from dark fermentation is the use of novel nanomaterials, such as Au, Ni, Ag, Cu, Fe, Pd, TiO2 and activated carbon [119][56]. In a study by Zhang et al. [115][57], the addition of cobalt ferrate nanoparticles was found to be effective in increasing biohydrogen yield from the dark fermentation of glucose.
Although there have been many studies on biohydrogen production from biomass via dark fermentation, a few challenges still exist in scaling up this technology. The interaction between different types of nanoparticles and the microbial community in dark fermentation systems should be further studied. It would be beneficial to see more work on techno-economic analyses, sustainability analyses and lifecycle assessments to help guide the industrial-scale designs of bioreactor systems for biohydrogen production.
5. Biodiesel
Biodiesel is a fatty acid methyl ester derived from vegetable oils, grease, animal fats, algae, microbial and other lipid sources. The production of biodiesel is a multi-step process that involves the extraction of oil, esterification, transesterification and purification. It has emerged as an alternative drop-in biofuel with enormous potential to reduce greenhouse gas emissions associated with fossil-derived diesel fuel. Biodiesel can also be used in existing diesel engines without requiring any major modifications [120][58].
Biodiesel production starts with the extraction of oil from the feedstock source via different methods, such as mechanical pressing, solvent extraction, microwave extraction, ultrasonic extraction and supercritical fluid extraction [121][59]. These oils are utilized as feedstock to produce biodiesel through transesterification in the presence of methanol and different acid catalysts. The most common raw materials used are vegetable oils, such as palm oil, soybean oil, corn oil, grapeseed, cottonseed oil, sunflower oil and canola oil [122][60]. Recent research advancements have shown promising results from algae as a potential biodiesel feedstock, which not only has the least competition to arable lands but also contributes to carbon fixation and wastewater reclamation [123][61]. The second step in biodiesel production is transesterification, which involves the reaction of the oil with an alcohol, typically methanol or ethanol, in the presence of a catalyst. The interaction of alcohol and oil produces fatty acid methyl esters, which are the main components of biodiesel. The final step in biodiesel production is the purification of impurities from biodiesel. This process is typically carried out by washing the biodiesel with water or using a dry-washing process [124,125][62][63].
Biodiesel production using lipases as biocatalysts has been the subject of numerous studies since it offers several advantages, such as high specificity, mild reaction conditions and environmentally benign process [123][61]. Lipases function as transesterification catalysts rather than hydrolases, which hydrolyze ester bonds. They facilitate the reaction between triglycerides and alcohol, leading to the production of biodiesel and glycerol. In addition to lipases, other enzymes, such as proteases and cellulases can also be employed in biodiesel production, although they are less commonly used compared to lipases [126][64]. These enzymes can be used to treat feedstocks that contain impurities like proteins or cellulosic material, which can interfere with the transesterification process. Lipases from microbial sources, such as bacteria, fungi and yeast have been extensively studied [127,128,129][65][66][67]. For example, lipases from Candida antarctica, Rhizopus oryzae and Pseudomonas cepacia have shown promising results in terms of activity and stability in biodiesel production [130][68].
Wang et al. [132][69] developed an enzyme-based pathway to convert crude algal oil into fatty acid methyl esters. The researchers used immobilized lipase from C. antarctica for biodiesel production. Furthermore, the authors enlightened the efficacy of different solvents, reaction times and temperatures in biodiesel conversion and recorded a 99.1% efficiency under optimized conditions (i.e., algal oil/tert-butanol ratio of 1:1, temperature of 25 °C and reaction time of 4 h). The authors also reported high stability for the lipase to withstand 41 cycles with minimal energy requirement and reduced wastewater discharge.
Jayaraman et al. [138][70] reported the efficacy of lipase-based catalysts in the enzymatic production of biodiesel. The authors used waste cooking oil as the source material to achieve 100% conversion to biodiesel with an optimized enzyme concentration of 1.5%, methanol as the solvent and 4 h. Sivaramakrishnan et al. [131][71] studied the efficacy of two microalgae (i.e., Chlorella and Scenedesmus) for methyl ester production via various solvent systems and cell disruption techniques.
Taher et al. [123][61] demonstrated biodiesel production via supercritical CO2 extraction from Nannochloropsis gaditana. The authors reported a 10.5% internal rate of return with a net present value of USD $8.31 million. They found that the transportation of equipment and materials is a significant contributor, with a share of 75% of the total impact. This can be linked to the use of fossil fuels for transportation. The lifecycle profiles are not only oriented with biocatalyst reactions but also importantly impacted with the downstream processes responsible for the purification and obtaining of the desired products.
References
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Butanol and ethanol production from lignocellulosic feedstock: Biomass pretreatment and bioconversion. Energy Sci. Eng. 2014, 2, 138–148.
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941.
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A review of biomass resources and thermochemical conversion technologies. Chem. Eng. Technol. 2022, 45, 791–799.
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633.
- Gopinarayanan, V.E.; Nair, N.U. Pentose metabolism in Saccharomyces cerevisiae: The need to engineer global regulatory systems. Biotechnol. J. 2019, 14, e1800364.
- Donato, P.D.; Finore, I.; Poli, A.; Nicolaus, B.; Lama, L. The production of second generation bioethanol: The biotechnology potential of thermophilic bacteria. J. Clean. Prod. 2019, 233, 1410–1417.
- Mladenovska, Z.; Dabrowski, S. Thermophilic Fermentative Bacterium Producing Butanol and/or Hydrogen from Glycerol. World Intellectual Property Organization International Patent Application NO. WO2010031793A2, 25 March 2010.
- Singh, N.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. Bioethanol production from pretreated whole slurry rice straw by thermophilic co-culture. Fuel 2021, 301, 121074.
- Zuliani, L.; Serpico, A.; De Simone, M.; Frison, N.; Fusco, S. Biorefinery gets hot: Thermophilic enzymes and microorganisms for second-generation bioethanol production. Processes 2021, 9, 1583.
- Jeffries, T.W. Ethanol fermentation on the move. Nat. Biotechnol. 2005, 23, 40–41.
- Raud, M.; Rocha-Meneses, L.; Lane, D.J.; Sippula, O.; Shurpali, N.J.; Kikas, T. Utilization of barley straw as feedstock for the production of different energy vectors. Processes 2021, 9, 726.
- Khoshkho, S.M.; Mahdavian, M.; Karimi, F.; Karimi-Maleh, H.; Razaghi, P. Production of bioethanol from carrot pulp in the presence of Saccharomyces cerevisiae and beet molasses inoculum; a biomass-based investigation. Chemosphere 2022, 286, 131688.
- Periyasamy, S.; Isabel, J.B.; Kavitha, S.; Karthik, V.; Mohamed, B.A.; Gizaw, D.G.; Sivashanmugam, P.; Aminabhavi, T.M. Recent advances in consolidated bioprocessing for conversion of lignocellulosic biomass into bioethanol—A review. Chem. Eng. J. 2023, 453, 139783.
- Qu, C.; Dai, K.; Fu, H.; Wang, J. Enhanced ethanol production from lignocellulosic hydrolysates by Thermoanaerobacterium aotearoense SCUT27/ΔargR1864 with improved lignocellulose-derived inhibitors tolerance. Renew. Energy 2021, 173, 652–661.
- Raita, M.; Ibenegbu, C.; Champreda, V.; Leak, D.J. Production of ethanol by thermophilic oligosaccharide utilising Geobacillus thermoglucosidasius TM242 using palm kernel cake as a renewable feedstock. Biomass Bioenergy 2016, 95, 45–54.
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30.
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Selvakumari, I.A.E.; Bharathiraja, B. Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour. Technol. 2019, 293, 122060.
- Tse, T.J.; Wiens, D.J.; Reaney, M.J. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268.
- Plaza, P.E.; Gallego-Morales, L.J.; Peñuela-Vásquez, M.; Lucas, S.; García-Cubero, M.T.; Coca, M. Biobutanol production from brewer’s spent grain hydrolysates by Clostridium beijerinckii. Bioresour. Technol. 2017, 244, 166–174.
- Nanda, S.; Golemi-Kotra, D.; McDermott, J.C.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Fermentative production of butanol: Perspectives on synthetic biology. New Biotechnol. 2017, 37, 210–221.
- Liao, C.; Seo, S.O.; Lu, T. System-level modeling of acetone–butanol–ethanol fermentation. FEMS Microbiol. Lett. 2016, 363, fnw074.
- Li, S.; Huang, L.; Ke, C.; Pang, Z.; Liu, L. Pathway dissection, regulation, engineering and application: Lessons learned from biobutanol production by solventogenic clostridia. Biotechnol. Biofuels 2020, 13, 39.
- Sonomoto, K.; Oshiro, M.; Hanada, K. Method of Producing Butanol. U.S. Patent US8420359B2, 16 April 2013.
- Wen, Z.; Minton, N.P.; Zhang, Y.; Li, Q.; Liu, J.; Jiang, Y.; Yang, S. Enhanced solvent production by metabolic engineering of a twin-clostridial consortium. Metab. Eng. 2017, 39, 38–48.
- Tsai, T.Y.; Lo, Y.C.; Dong, C.D.; Nagarajan, D.; Chang, J.S.; Lee, D.J. Biobutanol production from lignocellulosic biomass using immobilized Clostridium acetobutylicum. Appl. Energy 2020, 277, 115531.
- Allen, S.D.; Rusnack, M.R. Process for Improving the Yield and Efficiency of an Ethanol Fermentation Plant. U.S. Patent 8,927,239 B2, 6 January 2015.
- Josse, J.C.; Benedek, A. Syngas Biomethanation Process and Anaerobic Digestion System. U.S. Patent 9.284,203 B2, 15 March 2016.
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production—A review. Bioresour. Technol. 2018, 248, 29–36.
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and interspecies electron transfer in methanogenic microbial communities. Microbiology 2020, 89, 129–147.
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137.
- Latifi, P.; Karrabi, M.; Danesh, S. Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renew. Sustain. Energy Rev. 2019, 107, 288–296.
- Elsayed, M.; Diab, A.; Soliman, M. Methane production from anaerobic co-digestion of sludge with fruit and vegetable wastes: Effect of mixing ratio and inoculum type. Biomass Convers. Biorefin. 2021, 11, 989–998.
- Riggio, V.; Comino, E.; Rosso, M. Energy production from anaerobic co-digestion processing of cow slurry, olive pomace and apple pulp. Renew. Energy 2015, 83, 1043–1049.
- Adeleye, T.; Yeo, H.; Seth, R.; Hafez, H.; Biswas, N. Influence of mix ratio of potato peel and pig manure on reaction kinetics and methane recovery from anaerobic co-digestion. Can. J. Civ. Eng. 2022, 49, 675–682.
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537.
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035.
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74.
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330.
- Nanda, S.; Rana, R.; Zheng, Y.; Kozinski, J.A.; Dalai, A.K. Insights on pathways for hydrogen generation from ethanol. Sustain. Energy Fuels 2017, 1, 1232–1245.
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655.
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Fact. 2022, 21, 166.
- Sarangi, P.K.; Nanda, S. Biohydrogen production through dark fermentation. Chem. Eng. Technol. 2020, 43, 601–612.
- Lopez-Hidalgo, A.M.; Smoliński, A.; Sanchez, A. A meta-analysis of research trends on hydrogen production via dark fermentation. Int. J. Hydrogen Energy 2022, 47, 13300–13339.
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 978907.
- Laurent, B.; Serge, H.; Julien, M.; Christopher, H.; Philippe, T. Effects of hydrogen partial pressure on fermentative biohydrogen production by a chemotropic Clostridium bacterium in a new horizontal rotating cylinder reactor. Energy Proc. 2012, 29, 34–41.
- Kim, D.H.; Yoon, J.J.; Kim, S.H.; Park, J.H. Acceleration of lactate-utilizing pathway for enhancing biohydrogen production by magnetite supplementation in Clostridium butyricum. Bioresour. Technol. 2022, 359, 127448.
- Mohanraj, S.; Pandey, A.; Mohan, S.V.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Metabolic engineering and molecular biotechnology of biohydrogen production. In Biohydrogen, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–434.
- Schut, G.J.; Adams, M.W.W. The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: A new perspective on anaerobic hydrogen production. J. Bacteriol. 2009, 191, 4451–4457.
- Losey, N.A.; Mus, F.; Peters, J.W.; Le, H.M.; McInerney, M.J. Syntrophomonas wolfei uses an NADH-dependent, ferredoxin-independent -hydrogenase to reoxidize NADH. Environ. Microbiol. 2017, 83, e01335-17.
- Willquist, K.; Zeidan, A.A.; van Niel, E.W. Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: An efficient hydrogen cell factory. Microb. Cell Fact. 2010, 9, 89.
- Pradhan, N.; Dipasquale, L.; d’Ippolito, G.; Panico, A.; Lens, P.N.L.; Esposito, G.; Fontana, A. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600.
- Auria, R.; Boileau, C.; Davidson, S.; Casalot, L.; Christen, P.; Liebgott, P.P.; Combet-Blanc, Y. Hydrogen production by the hyperthermophilic bacterium Thermotoga maritima Part II: Modeling and experimental approaches for hydrogen production. Biotechnol. Biofuels 2016, 9, 268.
- Byrne, E.; Björkmalm, J.; Bostick, J.P.; Sreenivas, K.; Willquist, K.; van Niel, E.W.J. Characterization and adaptation of Caldicellulosiruptor strains to higher sugar concentrations, targeting enhanced hydrogen production from lignocellulosic hydrolysates. Biotechnol. Biofuels 2021, 14, 210.
- Bielen, A.A.M.; Verhaart, M.R.A.; van der Oost, J.; Kengen, S.W.M. Biohydrogen production by the thermophilic bacterium Caldicellulosiruptor saccharolyticus: Current status and perspectives. Life 2013, 3, 52–85.
- Li, X.; Sui, K.; Zhang, J.; Liu, X.; Xu, Q.; Wang, D.; Yang, Q. Revealing the mechanisms of rhamnolipid enhanced hydrogen production from dark fermentation of waste activated sludge. Sci. Total Environ. 2022, 806, 150347.
- Pugazhendhi, A.; Shobana, S.; Nguyen, D.D.; Banu, J.R.; Sivagurunathan, P.; Chang, S.W.; Ponnusamy, V.K.; Kumar, G. Application of nanotechnology (nanoparticles) in dark fermentative hydrogen production. Int. J. Hydrogen Energy 2019, 44, 1431–1440.
- Zhang, J.; Li, W.; Yang, J.; Li, Z.; Zhang, J.; Zhao, W.; Zang, L. Cobalt ferrate nanoparticles improved dark fermentation for hydrogen evolution. J. Clean. Prod. 2021, 316, 128275.
- Gouda, S.P.; Dhakshinamoorthy, A.; Rokhum, S.L. Metal-organic framework as a heterogeneous catalyst for biodiesel production: A review. Chem. Eng. J. Adv. 2022, 2022, 100415.
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021, 74, 105584.
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523.
- Taher, H.; Giwa, A.; Abusabiekeh, H.; Al-Zuhair, S. Biodiesel production from Nannochloropsis gaditana using supercritical CO2 for lipid extraction and immobilized lipase transesterification: Economic and environmental impact assessments. Fuel Process. Technol. 2020, 198, 106249.
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120.
- Khandelwal, K.; Boahene, P.; Nanda, S.; Dalai, A.K. Hydrogen production from supercritical water gasification of model compounds of crude glycerol from biodiesel industries. Energies 2023, 16, 3746.
- El-Bakry, M.; Abraham, J.; Cerda, A.; Barrena, R.; Ponsá, S.; Gea, T.; Sánchez, A. From wastes to high value added products: Novel aspects of SSF in the production of enzymes. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1999–2042.
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368.
- Bhan, C.; Singh, J. Role of microbial lipases in transesterification process for biodiesel production. Environ. Sustain. 2020, 3, 257–266.
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169.
- Santos, S.; Puna, J.; Gomes, J. A review on bio-based catalysts (immobilized enzymes) used for biodiesel production. Energies 2020, 13, 3013.
- Wang, Y.; Liu, J.; Gerken, H.; Zhang, C.; Hu, Q.; Li, Y. Highly-efficient enzymatic conversion of crude algal oils into biodiesel. Bioresour. Technol. 2014, 172, 143–149.
- Jayaraman, J.; Alagu, K.; Appavu, P.; Joy, N.; Jayaram, P.; Mariadoss, A. Enzymatic production of biodiesel using lipase catalyst and testing of an unmodified compression ignition engine using its blends with diesel. Renew. Energy 2020, 145, 399–407.
- Sivaramakrishnan, R.; Incharoensakdi, A. Production of methyl ester from two microalgae by two-step transesterification and direct transesterification. Environ. Sci. Pollut. Res. 2017, 24, 4950–4963.
More
