Phytoestrogens are plant-derived bioactive compounds with estrogen-like properties.
Their potential health benefits, especially in cancer prevention and treatment, have been a subject
of considerable research in the past decade. Phytoestrogens exert their effects, at least in part,
through interactions with estrogen receptors (ERs), mimicking or inhibiting the actions of natural
estrogens. Recently, there has been growing interest in exploring the impact of phytoestrogens on
osteosarcoma (OS), a type of bone malignancy that primarily affects children and young adults and
is currently presenting limited treatment options. Considering the critical role of the estrogen/ERs
axis in bone development and growth, the modulation of ERs has emerged as a highly promising
approach in the treatment of OS. This review provides an extensive overview of current literature
on the effects of phytoestrogens on human OS models. It delves into the multiple mechanisms
through which these molecules regulate the cell cycle, apoptosis, and key pathways implicated in
the growth and progression of OS, including ER signaling. Moreover, potential interactions between
phytoestrogens and conventional chemotherapy agents commonly used in OS treatment will be
examined. Understanding the impact of these compounds in OS holds great promise for developing
novel therapeutic approaches that can augment current OS treatment modalities.
Phytoestrogens are plant-derived bioactive compounds with estrogen-like properties. Their potential health benefits, especially in cancer prevention and treatment, have been a subject of considerable research in the past decade. Phytoestrogens exert their effects, at least in part, through interactions with estrogen receptors (ERs), mimicking or inhibiting the actions of natural estrogens. There has been growing interest in exploring the impact of phytoestrogens on osteosarcoma (OS), a type of bone malignancy that primarily affects children and young adults and is currently presenting limited treatment options. Considering the critical role of the estrogen/ERs axis in bone development and growth, the modulation of ERs has emerged as a highly promising approach in the treatment of OS. It delves into the multiple mechanisms through which these molecules regulate the cell cycle, apoptosis, and key pathways implicated in the growth and progression of OS, including ER signaling. Moreover, potential interactions between phytoestrogens and conventional chemotherapy agents commonly used in OS treatment will be examined. Understanding the impact of these compounds in OS holds great promise for developing novel therapeutic approaches that can augment current OS treatment modalities.
- osteosarcoma
- phytoestrogens
- anticancer effects
1. Introduction
2. Phytoestrogens: Chemical Classification and General Aspects
Phytoestrogens are produced by plants (more than 300 various species) as secondary metabolites which play crucial roles in various plant functions, such as defense against pathogens, pigmentation and protection from UV radiation, photosynthetic stress, and reactive oxygen species [16]. The quantity of phytoestrogens produced by a plant increases significantly during extreme growing conditions [17]. The human diet is rich in plant-containing phytoestrogens (i.e., vegetables, legumes, cereals, fruits, nuts, etc.), and beverages, such as wine, cider, beer, tea, and many more. Many edible plants contain multiple classes of phytoestrogens, adding to their diversity and potential health benefits [9]. Regarding their structural features, phytoestrogens represent a large and heterogenic class of non-steroidal substances characterized by a close structural similarity to the principal mammalian estrogen 17β-estradiol (E2) [11,12][11][12]. Shared structures include a phenolic ring and a pair of hydroxyl groups in opposite positions on the molecule (as in the case of E2 molecule), which are responsible for the interaction of phytoestrogens with the ligand-binding domain of ERα and ERβ. The exact position and number of these hydroxyl substituents is crucial in determining the binding affinity for the ERs and the activation of hormonal signaling [18,19][18][19]. The estrogenic or antiestrogenic properties of phytoestrogens in the target cells depend on their phenolic ring [20]. The phytoestrogens have been categorized into two main groups: flavonoids and non-flavonoids based on their chemical structure and properties. The classification and the basic structures of the most representative dietary phytoestrogens are illustrated in Figure 1.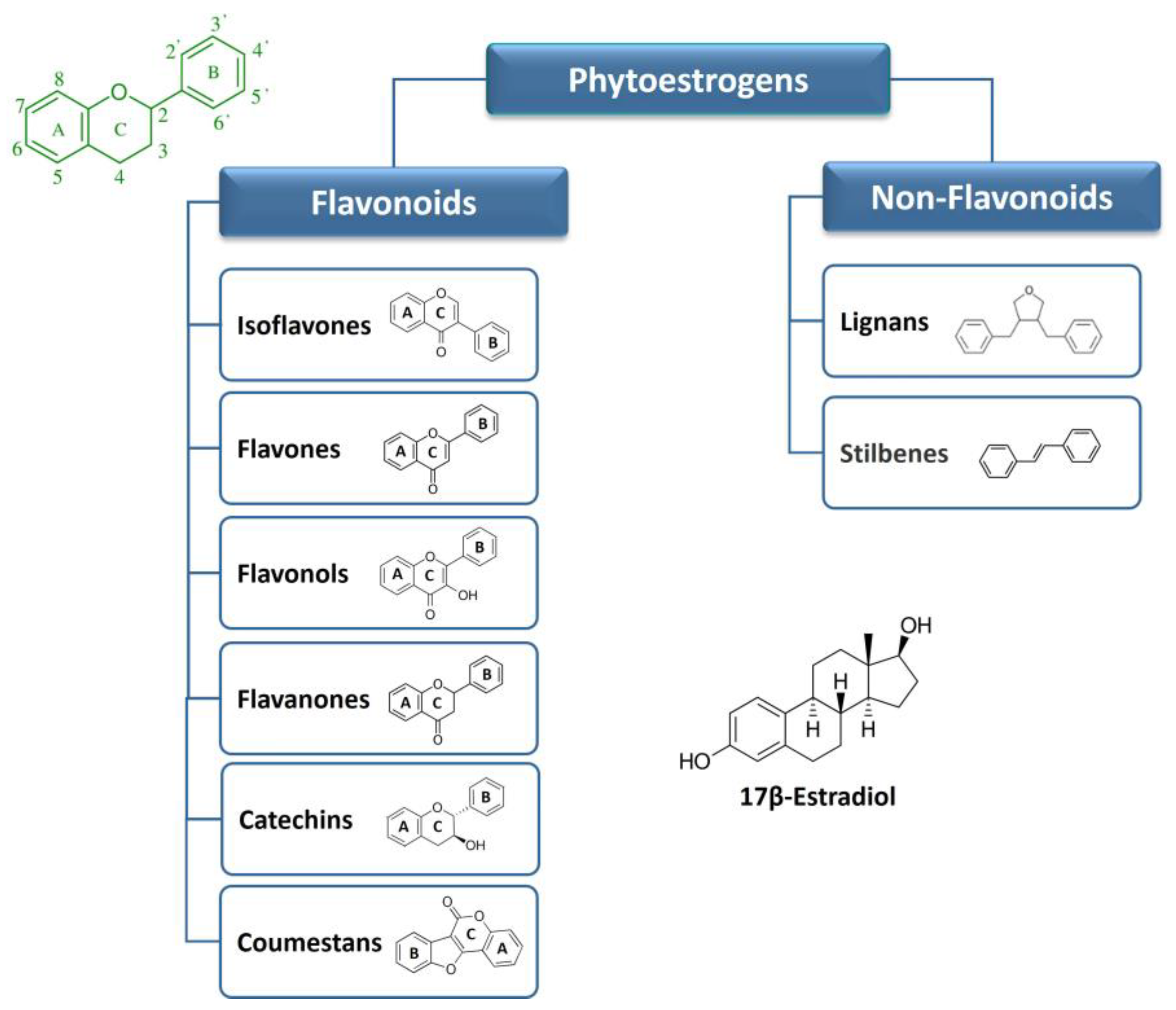
2.1. Flavonoids
Flavonoids are a large group of substituted phenolic compounds [22][21]. They share a common structure consisting of a fifteen-carbon skeleton composed of two benzene rings (A and B) connected by a heterocyclic pyran structure (C) in a C6–C3–C6 arrangement, as illustrated in Figure 1 [23][22]. The basic flavonoid skeleton can have numerous substituents, including hydroxyl groups typically found at positions 4′, 5′, and 7. Flavonoids can be further classified into different subclasses. Specifically, isoflavones are flavonoids, where the B ring is connected to the heterocyclic ring at the C3 position. On the other hand, flavonoids in which the B ring is linked at position 2 are divided into several subgroups, namely flavones, flavonols, flavanones, and catechins, depending on the degree of saturation and oxidation of the C ring (Figure 1). Coumestans are a distinct flavonoid which are characterized by a 1-benzoxolo (3,2-c)chromen-6-one structure formed by a benzoxole fused with a chromen-2-one [8]. The presence and position of hydroxyl groups and/or additional substituents contribute to the diversity of flavonoids and their biological activities [23,24,25][22][23][24]. Moreover, the addition of lipophilic prenyl side-chains can occur at different positions of the flavonoid skeleton, resulting in various prenylated derivatives with improved bioactivities and higher affinity to biological membranes. Prenylated flavonoids are much less common than flavonoids in nature [26][25]. Flavonoids are some of the most prevalent compounds found in fruits, vegetables, legumes, and tea and are generally concentrated in the fruit skin, bark, and flowers of plants [21][26]. Certain flavonoids, such as the flavonol quercetin, are found in all plant products (i.e., fruit, vegetables, cereals, leguminous plants, tea, and wine), but others are specific components of particular foods (i.e., flavanones in citrus fruit and isoflavones in soya). In most cases, food contains complex mixtures of flavonoids; for many food products, the composition is less known (for review, see [27]). In plants, most flavonoids are conjugated with one or more sugar residues linked to hydroxyl groups or aromatic carbons, so they mainly exist as glycosides [28].2.2. Non-Flavonoids
Non-flavonoids encompass a broad range of plant compounds that do not possess the characteristic flavonoid structure. Their structure consists of phenolic acids in either C6–C1 (benzoic acid) or C6–C3 (cinnamic acid) conformations and are mainly represented by lignans and stilbenes (Figure 1). Non-flavonoids may occur in the form of aglycones and glycosides [28]. Lignans are dimers of phenylpropanoid units connected via two specific carbons (C-2–C-2′) and are typically found in plant cell walls [29]. They are widespread and their content is high in common foods, including grains, nuts, seeds, vegetables, and drinks such as tea, coffee, or wine. Plant lignans are the principal source of dietary phytoestrogens in the Western diet [30]. Compounds, such as pinoresinol, lariciresinol, sesamin, enterolactone, and enterodiol can be found in this group [29]. Stilbenes are among the most relevant non-flavonoid phytoestrogens which consist of a 1,2-diphenylethylene nucleus that generates two isomers (cis and trans), with the trans-isomer being the most stable and biologically active [31,32][31][32]. More than 400 stilbene compounds have been identified in plants, with various structures ranging from monomers to octamers with different substituents, such as glycosyl, hydroxyl, methyl, or isopropyl radicals [31]. Monomeric stilbenes have been studied the most. These include resveratrol and polydatin (Section 7). They are naturally occurring in fruits, mostly in grapes, berries, and peanuts [33]. In general, the occurrence of stilbenes in the human diet is limited but represents an important part of phytoestrogen intake by people who follow a Mediterranean diet or who regularly drink wines.2.3. Metabolism of Dietary Phytoestrogens
Each class of dietary phytoestrogens has its own structural particularities, and studies regarding their bioavailability and metabolism are still far from being completed. There is no relation between the quantity of phytoestrogens in food and their bioavailability in the human body. Indeed, the rate and extent of absorption of dietary phytoestrogens in the intestine is determined primarily by their chemical structure and by factors such as molecular size and solubility, extent of glycosylation, hydroxylation, acylation, and degree of polymerization [12,34][12][34]. Most ingested phytoestrogens (e.g., isoflavones, lignans, and stilbenes), are predominantly present as estrogenically inactive glycosides in plant material [35]. After ingestion, phytoestrogens undergo extensive metabolization mediated both by tissue enzymes and gut microbiota, either prior to absorption or during enterohepatic circulation. The intestinal flora is capable of transforming aglycones into bioactive metabolites that are more similar to estrogens, being able to interfere with the endogenous estrogen signaling and associated cellular processes. In some cases, these metabolites have greater biological activities and different impacts on targeted tissues than their parent precursors [36,37][36][37]. For a detailed background on the absorption and metabolism of different phytoestrogens, see ref. [38]. Thus, individual variability in gut microbiota can influence the metabolism of these estrogenic molecules, contributing to their intake and beneficial effects [39]. Consequently, the identification, quantification, and individual differences among phytoestrogen metabolites are important issues when researching the health effects of phytoestrogens in humans.3. Phytoestrogen Mechanisms of Action—Anticancer Related Effects
In recent years, significant efforts have been made to elucidate the molecular mechanisms underlying the biological effects of phytoestrogens in both physiological and pathological conditions. The bioavailability and metabolism of phytoestrogens, as well as their effects on enzymes, nuclear receptors, and intracellular transduction mechanisms, play a crucial role in determining the overall impact of these compounds on cancer risk and progression [8]. However, the debate surrounding these effects persists, and further clarification is still needed. Phytoestrogens show a complex mode of action via interaction with the ER subtypes (i.e., ERα and ERβ), acting as either estrogen, triggering receptor pathways, or anti-estrogens, blocking normal estrogenic activity [10,40,41][10][40][41]. The dichotomy of ER modulating action induced by phytoestrogens led to the insertion of these compounds into the class of selective ER modulators (SERMs) [42,43,44,45][42][43][44][45] and probably provides an explanation regarding the conflicting evidence about the risks and benefits of these molecules on human health [46]. The activation of ER signaling pathways plays a vital role in the malignant progression of multiple cancers by comprehensively regulating downstream genes. The two ER subtypes have been described with different tissue distribution and ligand-binding affinities. ERα is mainly found in breast and uterine tissues and has been associated with pro-oncogenic responses while ERβ is the predominant isoform in the brain, bones, and blood vessels and is related to tumor-suppressive responses [19,47][19][47]. The alteration of the ERα/ERβ ratio in the affected tissues is one of the main reasons for the variability of estrogen-dependent cancer biology [48] and correlates with the response to the treatments and prognosis [49,50][49][50]. Phytoestrogens are known to bind ERs with much lower affinities than that of E2 (from 1/100 to 1/10,000), suggesting their weak estrogenic activities [19,47,51][19][47][51]. The actions of phytoestrogens via ERs can be mediated by genomic and/or non-genomic mechanisms, in a dose- and tissue-specific manner [52,56][52][53]. The ER-mediated genomic effects of phytoestrogens result in the regulation of target genes, which include anti-inflammatory, anti-apoptotic, metabolic, and mitochondrial genes, as well as an improvement in mitochondrial biogenesis and function, which leads to increased resistance to stress [57,58][54][55] (Figure 2).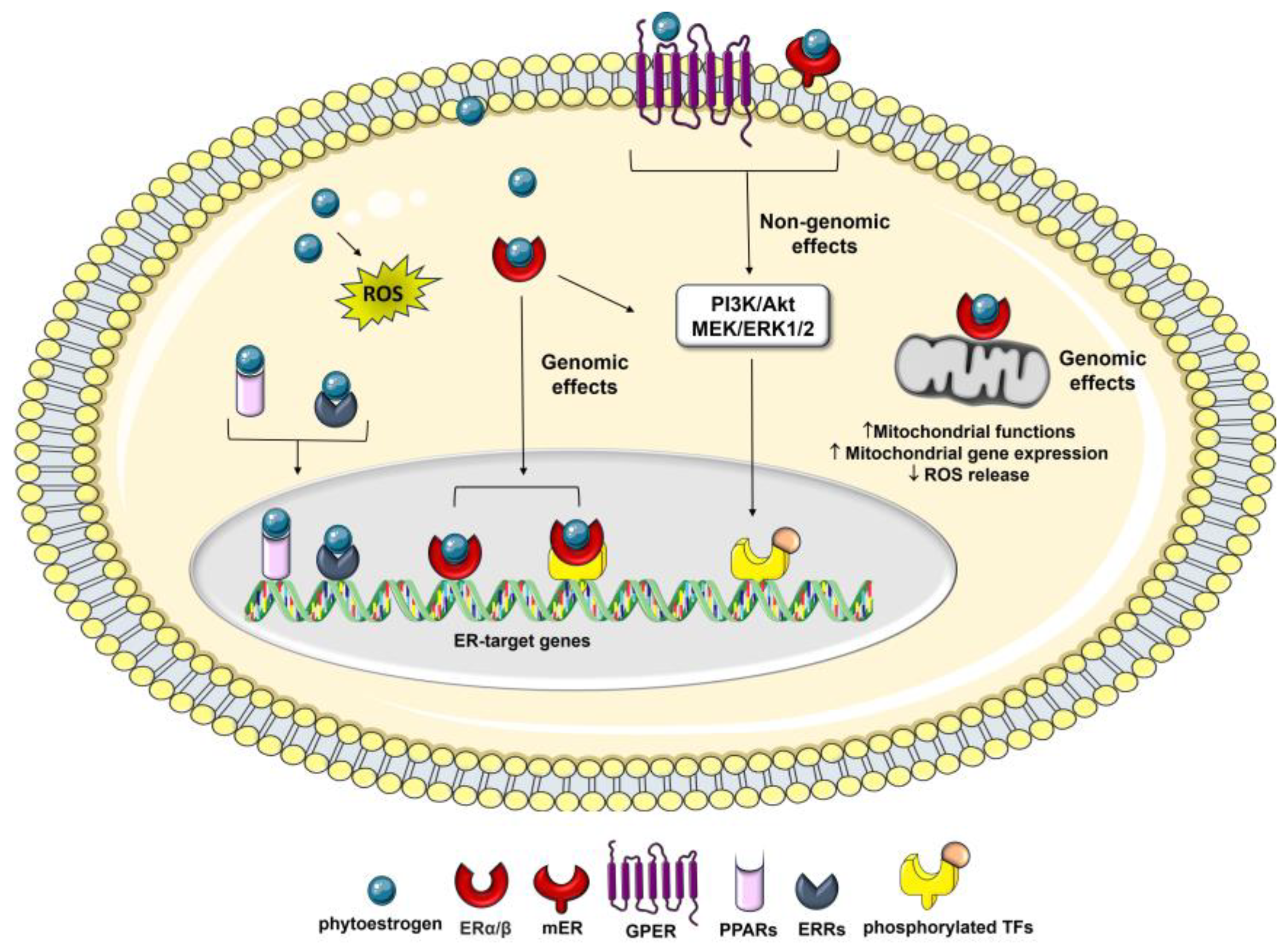
4. Molecular Basis of Osteosarcoma Pathogeneses
The difficulty in establishing an efficacious OS therapy is linked to the unclear specific markers for diagnosis and treatment. It is also due to the complexity of the OS genome, low incidence of this tumor, and significant biologic differences between OS subtypes. Nevertheless, the heterogeneity in the genotype of OS has translated into several expression profiles of macromolecular biomarkers, which are helpful in the clinic [2,80,81,82][2][77][78][79]. There are many genetic mutations observed in OS patients. The p53 and retinoblastoma (Rb) genes are well-known tumor-suppressor genes. Both germline and somatic mutations of the p53 and Rb genes have been proven to be involved in OS pathogenesis [82,83,84][79][80][81]. Inherited cancer predisposition syndromes, such as Li–Fraumeni, hereditary retinoblastoma, Rothmund–Thomson, Bloom, or Werner syndrome, may also influence the high appearance of this kind of tumor in young patients [83,85,86,87,88][80][82][83][84][85]. Among other genes mutated in more than 10% of OS cases, c-Myc plays a role in OS development and promotes cell invasion by activating MEK–ERK pathways. A high expression of c-Myc in OS tumors correlates with the formation of metastasis and poor prognosis [89][86]. Several studies have consistently demonstrated that OS cells have the capacity to develop and secrete a range of growth factors that exert autocrine and paracrine effects. Vascular endothelial growth factor (VEGF), transforming growth factor (TGF), IGF-I and IGF-II, and connective tissue growth factor (CTGF) are deregulated in OS, which leads to tumor progression and growth in target cells [82,90,91,92][79][87][88][89]. Parathyroid hormone-related peptide (PTHrP) and its receptor have also been implicated in OS progression and metastasis development, with PTHrP conferring OS chemoresistance by blocking signaling via p53 [93][90]. Epigenetic events have emerged as significant risk factors for OS, since the DNA methylation pattern of specific genes or gene regions and histone modifications may be involved in tumor development [94][91]. In addition, a variety of studies have found abnormally expressed levels of micro-RNAs (miRs), which have the potential to become prognostic biomarkers of OS. Overexpression of this molecule results in proliferation, migration, and invasion of tumor cells [68,95][65][92]. Among the miRNAs deregulated in osteosarcoma are miR-421, miR-16, miR-200b, and miR-101 [81,96,97][78][93][94]. OS is a highly metastatic tumor, and pulmonary metastases are the most common cause of death [82,98][79][95]. The ability of OS cells to metastasize has been found to be correlated with multiple processes and various cytophysiological changes, including changing the adhesion capabilities between cells and the extracellular matrix (ECM) and disrupting intercellular interactions [99,100][96][97]. Degradation of the ECM and components of the basement membrane caused by the concerted action of proteinases, such as matrix metalloproteinases (MMPs), cathepsins, and the plasminogen activator (PA), can play a critical role in OS invasion and metastasis [100][97]. Moreover, in metastatic forms of OS, some specific genetic changes have been observed, which include upregulation of the Wnt/β-catenin and Src pathways, the neurogenic locus notch homolog protein 1 and 2 (Notch1/Notch2) receptors [101,102][98][99] together with the downregulation of the Fas/Fas ligand pathway (a cell death pathway), which increases the metastatic potential of human OS [103,104][100][101]. In both primary bone cancer and bone metastases, the bone remodeling process creates a favorable environment for tumor establishment and progression. Osteoblasts and osteoclasts are the primary regulators of bone metabolism [105][102]. Specifically, osteoblasts secrete multiple components of ECM and MMPs in the OS niche, which are rich promoters of OS development. Moreover, osteoclasts play a pivotal role as bone-resorbing cells, and significant osteolysis exhibited in some OS cases can be directly attributed to the heightened activity of osteoclasts [100][97]. It has been demonstrated that OS is a condition characterized by deregulation in the signaling triad, i.e., the receptor activator of nuclear factor kB Ligand (RANKL), its receptors RANK, and osteoprotegerin (OPG) [106,107][103][104]. In its canonical function, RANKL, which is secreted by osteoblasts, induces bone destruction by mature osteoclasts. In response, osteoblasts secrete the OPG–RANKL decoy receptor and in this way inhibit osteoclast differentiation and resultant bone resorption [106,108][103][105]. The RANKL/OPG ratio in the blood is increased in high-grade OS, leading to the establishment of a vicious cycle between pathological bone remodeling and OS growth [108][105]. RANKL/RANK-signaling regulates OS cell migration and tissue-specific metastatic behavior in the lungs, but has no direct impact on OS-associated bone destruction and does not impact OS cell proliferation [106,109][103][106]. Thus, osteoclast pathways of differentiation, maturation, and activation constitute another compelling therapeutic target since the inhibition of bone resorption at the tumor–bone interface may lead to reduced local OS invasion [106][103]. Among the possible mechanisms that contribute to OS development in the bone microenvironment are alterations in the osteogenic pathway, which lead to the differentiation of mesenchymal stem cells (MSCs) into mature osteoblasts [81,110][78][107]. Defects in osteogenic differentiation or exposure to new non-native stimuli, such as pro-inflammatory cytokines and pro-tumor agents, may cause an imbalance between cell differentiation and proliferation, thus contributing to a malignant phenotype. OS cells share more characteristics with undifferentiated osteo-progenitors than with differentiated osteoblasts, including a high proliferative capacity and resistance to apoptosis. Indeed, osteogenic regulators associated with mature osteoblast phenotypes, such as CTGF, RUNX2, alkaline phosphatase (ALP), osteopontin (OPN), and osteocalcin (OCN), are very lowly expressed in both primary OS tumors and OS cell lines [111][108]. Although not well understood, some of the potential defects in the MSC differentiation cascade may include genetic and/or epigenetic changes in Wnt signaling, Rb, and p53. These alterations may lead to uncontrolled cell proliferation and disrupted differentiation, thus producing a tumorigenic phenotype [81,110][78][107]. Interestingly, treatments of human OS cells with therapeutic agents, such as peroxisome proliferator-activated receptor (PPAR) agonists [111][108], growth factors (e.g., PTHrP) [112][109], and SERMs [113][110], enable terminal differentiation and subsequent tumor inhibition. Hence, a better understanding of the relationship between defects in osteogenic differentiation and tumor development is of fundamental importance for the treatment of OS and promoting differentiation offers a potential for disease control.References
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. In Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O.S., Bielack, S., Eds.; Cancer Treatment and Research; Springer: Boston, MA, USA, 2009; Volume 152, pp. 3–13.
- Denduluri, S.; Wang, Z.; Yan, Z.; Wang, J.; Wei, Q.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; He, T.C. Molecular pathogenesis and therapeutic strategies of human osteosarcoma. J. Biomed. Res. 2015, 30, 5–18.
- Zhang, B.; Zhang, Y.; Li, R.; Li, J.; Lu, X.; Zhang, Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: A network meta-analysis. J. Orthop. Surg. Res. 2020, 15, 51.
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683.
- Arima, Y.; Nobusue, H.; Saya, H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020, 111, 2689–2695.
- Shukla, S.; Ohnuma, S.; Ambudkar, S.V. Improving Cancer Chemotherapy with Modulators of ABC Drug Transporters. Curr. Drug Targets 2011, 12, 621–630.
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15.
- Torrens-Mas, M.; Roca, P. Phytoestrogens for Cancer Prevention and Treatment. Biology 2020, 9, 427.
- Hu, X.J.; Song, W.R.; Gao, L.Y.; Nie, S.P.; Eisenbrand, G.; Xie, M.Y. Assessment of dietary phytoestrogen intake via plant-derived foods in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1325–1335.
- Cipolletti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the Antioxidant Activity of Dietary Polyphenols in Cancer: The Modulation of Estrogen Receptors (ERs) Signaling. Int. J. Mol. Sci. 2018, 19, 2624.
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phytoestrogens: End of a tale? Ann. Med. 2005, 37, 423–438.
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893.
- Yang, Z.-M.; Yang, M.-F.; Yu, W.; Tao, H.-M. Molecular mechanisms of estrogen receptor β-induced apoptosis and autophagy in tumors: Implication for treating osteosarcoma. J. Int. Med. Res. 2019, 47, 4644–4655.
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712.
- Tobeiha, M.; Rajabi, A.; Raisi, A.; Mohajeri, M.; Yazdi, S.M.; Davoodvandi, A.; Aslanbeigi, F.; Vaziri, M.; Hamblin, M.R.; Mirzaei, H. Potential of natural products in osteosarcoma treatment: Focus on molecular mechanisms. Biomed. Pharmacother. 2021, 144, 112257.
- Kondratyuk, T.P.; Pezzuto, J.M. Natural Product Polyphenols of Relevance to Human Health. Pharm. Biol. 2004, 42, 46–63.
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.; Wennberg, T.; Vuorela, H. Natural Products in the Process of Finding New Drug Candidates. Curr. Med. Chem. 2004, 11, 1375–1389.
- Miksicek, R.J. Estrogenic Flavonoids: Structural Requirements for Biological Activity. Exp. Biol. Med. 1995, 208, 44–50.
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263.
- Le Bail, J.-C.; Champavier, Y.; Chulia, A.-J.; Habrioux, G. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000, 66, 1281–1291.
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25.
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23.
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Xu, M.-J.; Wu, B.; Ding, T.; Chu, J.-H.; Li, C.-Y.; Zhang, J.; Wu, T.; Wu, J.; Liu, S.-J.; Liu, S.-L.; et al. Simultaneous characterization of prenylated flavonoids and isoflavonoids in Psoralea corylifolia L. by liquid chromatography with diode-array detection and quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2343–2358.
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251.
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917.
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333.
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2015, 2016, 3128951.
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340.
- Clavel, T.; Mapesa, J.O. Phenolics in Human Nutrition: Importance of the Intestinal Microbiome for Isoflavone and Lignan Bioavailability. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2433–2463.
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions. Nutrients 2019, 11, 1709.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907.
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636.
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236.
- Rietjens, I.M.C.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113.
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29.
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280.
- Jordan, V.C. SERMs: Meeting the promise of multifunctional medicines. J. Natl. Cancer Inst. 2007, 99, 350–356.
- Bedell, S.; Nachtigall, M.; Naftolin, F. The pros and cons of plant estrogens for menopause. J. Steroid Biochem. Mol. Biol. 2014, 139, 225–236.
- van de Schans, M.G.; Vincken, J.-P.; de Waard, P.; Hamers, A.R.; Bovee, T.F.; Gruppen, H. Glyceollins and dehydroglyceollins isolated from soybean act as SERMs and ER subtype-selective phytoestrogens. J. Steroid Biochem. Mol. Biol. 2016, 156, 53–63.
- Lee, G.-A.; Hwang, K.-A.; Choi, K.-C. Roles of Dietary Phytoestrogens on the Regulation of Epithelial-Mesenchymal Transition in Diverse Cancer Metastasis. Toxins 2016, 8, 162.
- McCarty, M.F. Isoflavones made simple-genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypotheses 2006, 66, 1093–1114.
- Thomas, C.; Gustafsson, J. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608.
- Jonsson, P.; Katchy, A.; Williams, C. Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells. Endocr.-Relat. Cancer 2013, 21, 143–160.
- Huang, B.; Warner, M.; Gustafsson, J.Å. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol. Cell. Endocrinol. 2015, 418, 240–244.
- van der Woude, H.; ter Veld, M.G.R.; Jacobs, N.; van der Saag, P.T.; Murk, A.J.; Rietjens, I.M.C.M. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005, 49, 763–771.
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667.
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870.
- Robb, E.L.; Stuart, J.A. Resveratrol interacts with estrogen receptor-β to inhibit cell replicative growth and enhance stress resistance by upregulating mitochondrial superoxide dismutase. Free Radic. Biol. Med. 2011, 50, 821–831.
- Surico, D.; Ercoli, A.; Farruggio, S.; Raina, G.; Filippini, D.; Mary, D.; Minisini, R.; Surico, N.; Pirisi, M.; Grossini, E. Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro. Cell. Physiol. Biochem. 2017, 42, 1051–1062.
- Tanwar, A.K.; Dhiman, N.; Kumar, A.; Jaitak, V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur. J. Med. Chem. 2021, 213, 113037.
- Yamaguchi, Y. Microenvironmental regulation of estrogen signals in breast cancer. Breast Cancer 2007, 14, 175–181.
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726.
- Viñas, R.; Jeng, Y.-J.; Watson, C.S. Non-Genomic Effects of Xenoestrogen Mixtures. Int. J. Environ. Res. Public Health 2012, 9, 2694–2714.
- Molina, L.; Bustamante, F.A.; Bhoola, K.D.; Figueroa, C.D.; Ehrenfeld, P. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin. Sci. 2018, 132, 2583–2598.
- Razandi, M.; Pedram, A.; Merchenthaler, I.; Greene, G.L.; Levin, E.R. Plasma membrane estrogen receptors exist and functions as dimers. Mol. Endocrinol. 2004, 18, 2854–2865.
- Márquez, D.C.; Lee, J.; Lin, T.; Pietras, R.J. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine 2001, 16, 073–082.
- Gong, P.; Madak-Erdogan, Z.; Flaws, J.A.; Shapiro, D.J.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells. Mol. Cell. Endocrinol. 2016, 437, 190–200.
- Huang, Q.; Chen, Q. Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids. PPAR Res. 2017, 2017, 3203161.
- Kumari, K.; Adhya, A.K.; Rath, A.K.; Reddy, P.B.; Mishra, S.K. Estrogen-related receptors alpha, beta and gamma expression and function is associated with transcriptional repressor EZH2 in breast carcinoma. BMC Cancer 2018, 18, 690.
- Barnes, S.; Zheng, X.; Lee, S.-K.; Song, W.O.; Chun, O.K.; Hwang, I.; Shin, H.S.; Kim, B.-G.; Kim, K.S.; Lee, S.-Y.; et al. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98.
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381.
- Pandima Devi, K.; Rajavel, T.; Daglia, M.; Nabavi, S.F.; Bishayee, A.; Nabavi, S.M. Targeting MiRNAs by Polyphenols: Novel Therapeutic Strategy for Cancer. Semin. Cancer Biol. 2017, 46, 146–157.
- Hsieh, C.-J.; Hsu, Y.-L.; Huang, Y.-F.; Tsai, E.-M. Molecular Mechanisms of Anticancer Effects of Phytoestrogens in Breast Cancer. Curr. Protein Pept. Sci. 2018, 19, 323–332.
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin. Cancer Biol. 2017, 46, 1–13.
- Park, C.; Cha, H.-J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.-W.; Han, M.H.; Choi, S.H.; et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327.
- Hsiao, Y.-C.; Peng, S.-F.; Lai, K.-C.; Liao, C.-L.; Huang, Y.-P.; Lin, C.-C.; Lin, M.-L.; Liu, K.-C.; Tsai, C.-C.; Ma, Y.-S.; et al. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol. 2019, 34, 443–456.
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77.
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306.
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216.
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216.
- Martin, J.W.; Squire, J.A.; Zielenska, M. The genetics of osteosarcoma. Sarcoma 2012, 2012, 627254.
- de Azevedo, J.W.V.; Fernandes, T.A.A.D.M.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araujo, J.M.G. Biology and pathogenesis of human osteosarcoma. Oncol. Lett. 2020, 19, 1099–1116.
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130.
- Hameed, M.; Mandelker, D. Tumor Syndromes Predisposing to Osteosarcoma. Adv. Anat. Pathol. 2018, 25, 217–222.
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112.
- Wang, L.L.; Gannavarapu, A.; Kozinetz, C.A.; Levy, M.L.; Lewis, R.A.; Chintagumpala, M.M.; Ruiz-Maldanado, R.; Contreras-Ruiz, J.; Cunniff, C.; Erickson, R.P.; et al. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J. Natl. Cancer Inst. 2003, 95, 669–674.
- Kleinerman, R.A.; Schonfeld, S.J.; Tucker, M.A. Sarcomas in hereditary retinoblastoma. Clin. Sarcoma Res. 2012, 2, 15.
- Rickel, K.; Fang, F.; Tao, J. Molecular genetics of osteosarcoma. Bone 2017, 102, 69–79.
- Fiedorowicz, M.; Bartnik, E.; Sobczuk, P.; Teterycz, P.; Czarnecka, A.M. Molecular biology of sarcoma. Oncol. Clin. Pract. 2019, 14, 307–330.
- Chen, D.; Zhao, Z.; Huang, Z.; Chen, D.-C.; Zhu, X.-X.; Wang, Y.-Z.; Yan, Y.-W.; Tang, S.; Madhavan, S.; Ni, W.; et al. Super enhancer inhibitors suppress MYC driven transcriptional amplification and tumor progression in osteosarcoma. Bone Res. 2018, 6, 11.
- Kim, L.C.; Song, L.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595.
- Hingorani, P.; Zhang, W.; Gorlick, R.; Kolb, E.A. Inhibition of Src Phosphorylation Alters Metastatic Potential of Osteosarcoma In Vitro but not In Vivo. Clin. Cancer Res. 2009, 15, 3416–3422.
- Broadhead, M.L.; Clark, J.C.M.; Myers, D.E.; Dass, C.R.; Choong, P.F.M. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011, 959248.
- Gagiannis, S.; Müller, M.; Uhlemann, S.; Koch, A.; Melino, G.; Krammer, P.H.; Nawroth, P.P.; Brune, M.; Schilling, T. Parathyroid hormone-related protein confers chemoresistance by blocking apoptosis signaling via death receptors and mitochondria. Int. J. Cancer 2009, 125, 1551–1557.
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36.
- DeRoo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570.
- Zhou, S.; Wang, B.; Hu, J.; Zhou, Y.; Jiang, M.; Wu, M.; Qin, L.; Yang, X. miR-421 is a diagnostic and prognostic marker in patients with osteosarcoma. Tumor Biol. 2016, 37, 9001–9007.
- Ren, Z.; He, M.; Shen, T.; Wang, K.; Meng, Q.; Chen, X.; Zhou, L.; Han, Y.; Ji, C.; Liu, S.; et al. MiR-421 promotes the development of osteosarcoma by regulating MCPIP1 expression. Cancer Biol. Ther. 2020, 21, 231–240.
- PosthumaDeBoer, J.; Witlox, M.A.; Kaspers, G.J.L.; van Royen, B.J. Molecular alterations as target for therapy in metastatic osteosarcoma: A review of literature. Clin. Exp. Metastasis 2011, 28, 493–503.
- Felx, M.; Guyot, M.-C.; Isler, M.; Turcotte, R.E.; Doyon, J.; Khatib, A.-M.; Leclerc, S.; Moreau, A.; Moldovan, F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin. Sci. 2006, 110, 645–654.
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178.
- Chen, C.; Zhao, M.; Tian, A.; Zhang, X.; Yao, Z.; Ma, X. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 2015, 6, 17570–17583.
- Fang, F.; VanCleave, A.; Helmuth, R.; Torres, H.; Rickel, K.; Wollenzien, H.; Sun, H.; Zeng, E.; Zhao, J.; Tao, J. Targeting the Wnt/β-catenin pathway in human osteosarcoma cells. Oncotarget 2018, 9, 36780–36792.
- Worth, L.L.; Lafleur, E.A.; Jia, S.-F.; Kleinerman, E.S. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol. Rep. 2002, 9, 823–827.
- Lafleur, E.A.; Koshkina, N.V.; Stewart, J.; Jia, S.-F.; Worth, L.L.; Duan, X.; Kleinerman, E.S. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin. Cancer Res. 2004, 10, 8114–8119.
- Alloisio, G.; Ciaccio, C.; Fasciglione, G.F.; Tarantino, U.; Marini, S.; Coletta, M.; Gioia, M. Effects of Extracellular Osteoanabolic Agents on the Endogenous Response of Osteoblastic Cells. Cells 2021, 10, 2383.
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10, 398.
- Nørregaard, K.S.; Jürgensen, H.J.; Gårdsvoll, H.; Engelholm, L.H.; Behrendt, N.; Søe, K. Osteosarcoma and Metastasis Associated Bone Degradation—A Tale of Osteoclast and Malignant Cell Cooperativity. Int. J. Mol. Sci. 2021, 22, 6865.
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor Activator of Nuclear Factor κB Ligand (RANKL)/Osteoprotegerin (OPG) Ratio Is Increased in Severe Osteolysis. Am. J. Pathol. 2003, 163, 2021–2031.
- Chen, Y.; Di Grappa, M.A.; Molyneux, S.D.; McKee, T.D.; Waterhouse, P.; Penninger, J.M.; Khokha, R. RANKL blockade prevents and treats aggressive osteosarcomas. Sci. Transl. Med. 2015, 7, 317ra197.
- Wagner, E.R.; Luther, G.; Zhu, G.; Luo, Q.; Shi, Q.; Kim, S.H.; Gao, J.-L.; Huang, E.; Gao, Y.; Yang, K.; et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma 2011, 2011, 325238.
- Wagner, E.R.; He, B.-C.; Chen, L.; Zuo, G.-W.; Zhang, W.; Shi, Q.; Luo, Q.; Luo, X.; Liu, B.; Luo, J.; et al. Therapeutic Implications of PPARgamma in Human Osteosarcoma. PPAR Res. 2010, 2010, 956427.
- Carpio, L.; Gladu, J.; Goltzman, D.; Rabbani, S.A.; Robichaux, W.G.; Cheng, X.; Agas, D.; Marchetti, L.; Capitani, M.; Sabbieti, M.G. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. Am. J. Physiol. Metab. 2001, 281, E489–E499.
- Kallio, A.; Guo, T.; Lamminen, E.; Seppänen, J.; Kangas, L.; Väänänen, H.K.; Härkönen, P. Estrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cells. Mol. Cell. Endocrinol. 2008, 289, 38–48.
