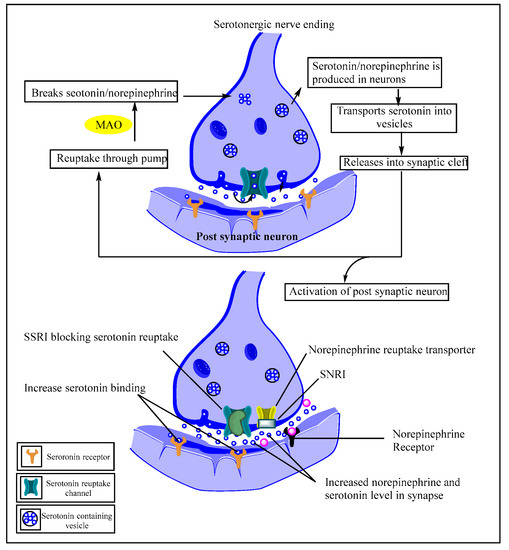
Serotoninergic signaling is identified as a crucial player in psychiatric disorders (notably depression), presenting it as a significant therapeutic target for treating such conditions. Inhibitors of serotoninergic signaling (especially selective serotonin reuptake inhibitors (SSRI) or serotonin and norepinephrine reuptake inhibitors (SNRI)) are prominently selected as first-line therapy for the treatment of depression, which benefits via increasing low serotonin levels and norepinephrine by blocking serotonin/norepinephrine reuptake and thereby increasing activity. While developing newer heterocyclic scaffolds to target/modulate the serotonergic systems, imidazole-bearing pharmacophores have emerged. The imidazole-derived pharmacophore already demonstrated unique structural characteristics and an electron-rich environment, ultimately resulting in a diverse range of bioactivities.
- imidazole
- drug discovery
- antidepressants
- serotonin reuptake inhibitors
- structure–activity relationship
1. Introduction
2. Etiology of Depression, Structural and Mechanistic Insights
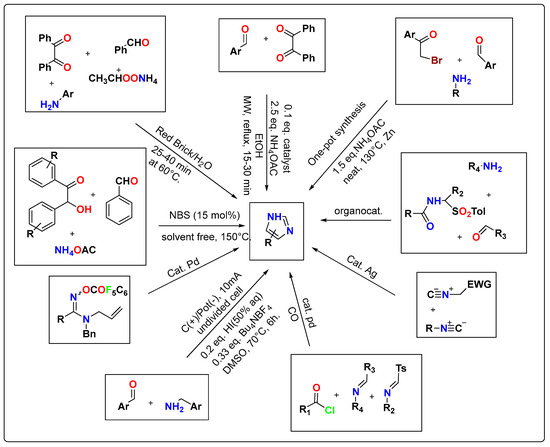
3. Modern Synthetic Methods for Substituted Imidazole Derivatives
References
- Whitaker-Azmitia, P.M. The Discovery of Serotonin and its Role in Neuroscience. Neuropsychopharmacology 1999, 21, 2–8.
- Sjoerdsma, A.; Palfreyman, M.G. History of serotonin and serotonin disorders. Ann. New York Acad. Sci. 1990, 600, 1–7.
- Andrews, A.M. Celebrating serotonin. ACS Chem. Neurosci. 2012, 3, 644–645.
- Maclean, J.A.; Schoenwaelder, S.M. Serotonin in platelets. In Serotonin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–119.
- Jonnakuty, C.; Gragnoli, C. What do we know about serotonin? J. Cell. Physiol. 2008, 217, 301–306.
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366.
- Kroeze, W.K.; Kristiansen, K.; Roth, B.L. Molecular biology of serotonin receptors-structure and function at the molecular level. Curr. Top. Med. Chem. 2002, 2, 507–528.
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol. Int. 2021, 13, 387–401.
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022.
- Kendrick, T.; Collinson, S. Antidepressants and the serotonin hypothesis of depression. BMJ 2022, 378, o1993.
- Bhutani, P.; Joshi, G.; Raja, N.; Bachhav, N.; Rajanna, P.K.; Bhutani, H.; Paul, A.T.; Kumar, R. U.S. FDA Approved Drugs from 2015–June 2020: A Perspective. J. Med. Chem. 2021, 64, 2339–2381.
- Hu, F.; Zhang, L.; Nandakumar, K.S.; Cheng, K. Imidazole Scaffold Based Compounds in the Development of Therapeutic Drugs. Curr. Top. Med. Chem. 2021, 21, 2514–2528.
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437.
- Hossain, M.; Nanda, A.K. A review on heterocyclic: Synthesis and their application in medicinal chemistry of imidazole moiety. Science 2018, 6, 83–94.
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 1–69.
- Demchenko, S.; Lesyk, R.; Yadlovskyi, O.; Zuegg, J.; Elliott, A.G.; Drapak, I.; Fedchenkova, Y.; Suvorova, Z.; Demchenko, A. Synthesis, antibacterial and antifungal activity of new 3-aryl-5H-pyrrolo imidazole and 5H-imidazo azepine quaternary salts. Molecules 2021, 26, 4253.
- Chahal, S.; Rani, P.; Kiran; Sindhu, J.; Joshi, G.; Ganesan, A.; Kalyaanamoorthy, S.; Mayank; Kumar, P.; Singh, R. Design and Development of COX-II Inhibitors: Current Scenario and Future Perspective. ACS Omega 2023, 8, 17446–17498.
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as potential anticancer agents. MedChemComm 2017, 8, 1742–1773.
- Dhameliya, T.M.; Patel, K.I.; Tiwari, R.; Vagolu, S.K.; Panda, D.; Sriram, D.; Chakraborti, A.K. Design, synthesis, and biological evaluation of benzo imidazole-2-carboxamides as new anti-TB agents. Bioorganic Chem. 2021, 107, 104538.
- Ali, E.M.; Abdel-Maksoud, M.S.; Hassan, R.M.; Mersal, K.I.; Ammar, U.M.; Se-In, C.; He-Soo, H.; Kim, H.-K.; Lee, A.; Lee, K.-T. Design, synthesis and anti-inflammatory activity of imidazol-5-yl pyridine derivatives as p38α/MAPK14 inhibitor. Bioorganic Med. Chem. 2021, 31, 115969.
- Eliewi, A.; Al-Garawi, Z.; Al-Kazzaz, F.; Atia, A. Multi target-directed imidazole derivatives for neurodegenerative diseases. J. Phys. Conf. Ser. 2021, 1853, 012066.
- Dhingra, A.K.; Chopra, B.; Jain, A.; Chaudhary, J. Imidazole: Multi-targeted therapeutic leads for the management of Alzheimer’s disease. Mini Rev. Med. Chem. 2022, 22, 1352–1373.
- Akhtar, A.; Gupta, S.M.; Dwivedi, S.; Kumar, D.; Shaikh, M.F.; Negi, A. Preclinical Models for Alzheimer’s Disease: Past, Present, and Future Approaches. ACS Omega 2022, 7, 47504–47517.
- Georgiou, N.; Gkalpinos, V.K.; Katsakos, S.D.; Vassiliou, S.; Tzakos, A.G.; Mavromoustakos, T. Rational Design and Synthesis of AT1R Antagonists. Molecules 2021, 26, 2927.
- Hassan, A.Y.; El-Sebaey, S.A.; El Deeb, M.A.; Elzoghbi, M.S. Potential antiviral and anticancer effect of imidazoles and bridgehead imidazoles generated by HPV-Induced cervical carcinomas via reactivating the P53/pRb pathway and inhibition of CA IX. J. Mol. Struct. 2021, 1230, 129865.
- Modh, P.G.; Patel, L.J. Synthesis, Drug Likeness and In-vitro Screening of Some Novel Quinazolinone Derivatives for Anti-Obesity Activity. J. Pharm. Res. Int. 2021, 33, 81–92.
- Adeyemi, O.S.; Eseola, A.O.; Plass, W.; Kato, K.; Otuechere, C.A.; Awakan, O.J.; Atolani, O.; Otohinoyi, D.A.; Elebiyo, T.C.; Evbuomwan, I.O. The anti-parasite action of imidazole derivatives likely involves oxidative stress but not HIF-1α signaling. Chem.-Biol. Interact. 2021, 349, 109676.
- Negi, A.; Alex, J.M.; Amrutkar, S.M.; Baviskar, A.T.; Joshi, G.; Singh, S.; Banerjee, U.C.; Kumar, R. Imine/amide–imidazole conjugates derived from 5-amino-4-cyano-N1-substituted benzyl imidazole: Microwave-assisted synthesis and anticancer activity via selective topoisomerase-II-α inhibition. Bioorganic Med. Chem. 2015, 23, 5654–5661.
- Cohen, N.A.; Stewart, M.L.; Gavathiotis, E.; Tepper, J.L.; Bruekner, S.R.; Koss, B.; Opferman, J.T.; Walensky, L.D. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem. Biol. 2012, 19, 1175–1186.
- Abou Samra, A.; Robert, A.; Gov, C.; Favre, L.; Eloy, L.; Jacquet, E.; Bignon, J.; Wiels, J.; Desrat, S.; Roussi, F. Dual inhibitors of the pro-survival proteins Bcl-2 and Mcl-1 derived from natural compound meiogynin A. Eur. J. Med. Chem. 2018, 148, 26–38.
- Litaudon, M.; Bousserouel, H.; Awang, K.; Nosjean, O.; Martin, M.-T.; Dau, M.E.T.H.; Hadi, H.A.; Boutin, J.A.; Sevenet, T.; Gueritte, F. A dimeric sesquiterpenoid from a Malaysian Meiogyne as a new inhibitor of Bcl-xL/BakBH3 domain peptide interaction. J. Nat. Prod. 2009, 72, 480–483.
- Negi, A.; Murphy, P.V. Natural products as Mcl-1 inhibitors: A comparative study of experimental and computational modelling data. Chemistry 2022, 4, 983–1009.
- Wang, G.; Wang, Y.; Wang, L.; Han, L.; Hou, X.; Fu, H.; Fang, H. Design, synthesis and preliminary bioactivity studies of imidazolidine-2, 4-dione derivatives as Bcl-2 inhibitors. Bioorganic Med. Chem. 2015, 23, 7359–7365.
- Negi, A.; Murphy, P.V. Development of Mcl-1 inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 210, 113038.
- Negi, A.; Ramarao, P.; Kumar, R. Recent advancements in small molecule inhibitors of insulin–like growth factor-1 receptor (IGF-1R) tyrosine kinase as anticancer agents. Mini Rev. Med. Chem. 2013, 13, 653–681.
- Peled, N.; Wynes, M.W.; Ikeda, N.; Ohira, T.; Yoshida, K.; Qian, J.; Ilouze, M.; Brenner, R.; Kato, Y.; Mascaux, C. Insulin-like growth factor-1 receptor (IGF-1R) as a biomarker for resistance to the tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. Cell. Oncol. 2013, 36, 277–288.
- Osmaniye, D.; Levent, S.; Sağlık, B.N.; Karaduman, A.B.; Özkay, Y.; Kaplancıklı, Z.A. Novel imidazole derivatives as potential aromatase and monoamine oxidase-B inhibitors against breast cancer. New J. Chem. 2022, 46, 7442–7451.
- Çetiner, G.; Çevik, U.A.; Celik, I.; Bostancı, H.E.; Özkay, Y.; Kaplancıklı, Z.A. New imidazole derivatives as aromatase inhibitor: Design, synthesis, biological activity, molecular docking, and computational ADME-Tox studies. J. Mol. Struct. 2023, 1278, 134920.
- Makar, S.; Saha, T.; Swetha, R.; Gutti, G.; Kumar, A.; Singh, S.K. Rational approaches of drug design for the development of selective estrogen receptor modulators (SERMs), implicated in breast cancer. Bioorganic Chem. 2020, 94, 103380.
- Xie, B.; Xu, B.; Xin, L.; Wei, Y.; Guo, X.; Dong, C. Discovery of estrogen receptor α targeting caged hypoxia-responsive PROTACs with an inherent bicyclic skeleton for breast cancer treatment. Bioorganic Chem. 2023, 137, 106590.
- Negi, A.; Kesari, K.K.; Voisin-Chiret, A.S. Estrogen Receptor-α Targeting: PROTACs, SNIPERs, Peptide-PROTACs, Antibody Conjugated PROTACs and SNIPERs. Pharmaceutics 2022, 14, 2523.
- Li, S.-R.; Tan, Y.-M.; Zhang, L.; Zhou, C.-H. Comprehensive insights into medicinal research on imidazole-based supramolecular complexes. Pharmaceutics 2023, 15, 1348.
- Ralhan, R.; Kaur, J. Alkylating agents and cancer therapy. Expert Opin. Ther. Pat. 2007, 17, 1061–1075.
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Ping Dou, Q. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825.
- Zhou, C.-H.; Zhang, Y.-Y.; Yan, C.-Y.; Wan, K.; Gan, L.-L.; Shi, Y. Recent researches in metal supramolecular complexes as anticancer agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2010, 10, 371–395.
- Liu, P.; Jia, J.; Zhao, Y.; Wang, K.-Z. Recent advances on dark and light-activated cytotoxity of imidazole-containing ruthenium complexes. Mini Rev. Med. Chem. 2016, 16, 272–289.
- Liu, C.; Yang, W.; Du, J.; Shen, P.; Yang, C. A Boron 2-(2′-pyridyl) Imidazole Fluorescence Probe for Bovine Serum Albumin: Discrimination over Other Proteins and Identification of Its Denaturation. Photochem. Photobiol. 2017, 93, 1414–1422.
- Liu, H.-Y.; Zhang, S.-Q.; Cui, M.-C.; Gao, L.-H.; Zhao, H.; Wang, K.-Z. pH-sensitive near-IR emitting dinuclear ruthenium complex for recognition, two-photon luminescent imaging, and subcellular localization of cancer cells. ACS Appl. Bio Mater. 2020, 3, 5420–5427.
- Zhu, Y.; Xu, C.; Wang, Y.; Chen, Y.; Ding, X.; Yu, B. Luminescent detection of the lipopolysaccharide endotoxin and rapid discrimination of bacterial pathogens using cationic platinum (ii) complexes. RSC Adv. 2017, 7, 32632–32636.
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.; Costa, S.P.; Raposo, M.M.; Martinez-Manez, R.; Sancenon, F. 4-(4, 5-Diphenyl-1H-imidazole-2-yl)-N, N-dimethylaniline-Cu (II) complex, a highly selective probe for glutathione sensing in water-acetonitrile mixtures. Dye. Pigment. 2018, 159, 45–48.
- Zhao, C.; Kong, X.; Shuang, S.; Wang, Y.; Dong, C. An anthraquinone-imidazole-based colorimetric and fluorescent sensor for the sequential detection of Ag+ and biothiols in living cells. Analyst 2020, 145, 3029–3037.
- Tian, F.; Jiang, X.; Dou, X.; Wu, Q.; Wang, J.; Song, Y. Design and synthesis of novel adenine fluorescence probe based on Eu (III) complexes with dtpa-bis (guanine) ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 194–200.
- Zhu, Y.-Y.; Sun, Q.; Shi, J.-W.; Xia, H.-Y.; Wang, J.-L.; Chen, H.-Y.; He, H.-F.; Shen, L.; Zhao, F.; Zhong, J. A novel triple substituted imidazole fluorescent sensor for Ag+ and its imaging in living cell and zebrafish. J. Photochem. Photobiol. A Chem. 2020, 389, 112244.
- Suresh, S.; Bhuvanesh, N.; Raman, A.; Sugumar, P.; Padmanabhan, D.; Easwaramoorthi, S.; Ponnuswamy, M.N.; Kavitha, S.; Nandhakumar, R. Experimental and theoretical studies of imidazole based chemosensor for Palladium and their biological applications. J. Photochem. Photobiol. A Chem. 2019, 385, 112092.
- Mehta, P.K.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Ratiometric detection of Cu+ in aqueous buffered solutions and in live cells using fluorescent peptidyl probe to mimic the binding site of the metalloprotein for Cu+. Sens. Actuators B Chem. 2018, 256, 393–401.
- Bhattacharya, A.; Mahata, S.; Bandyopadhyay, A.; Mandal, B.B.; Manivannan, V. Application of 2, 4, 5-tris (2-pyridyl) imidazole as ‘turn-off’fluorescence sensor for Cu (II) and Hg (II) ions and in vitro cell imaging. Luminescence 2022, 37, 883–891.
- Yin, H.; Cheng, F.; Liu, Z.; He, C.; Yang, Y.; Wang, K. Preparation, characterization, pH titration, and electrochemical properties of an anthracene-bridged binuclear ruthenium complex containing imidazole. J. Coord. Chem. 2019, 72, 2957–2967.
- Niu, L.; Liu, J.; Gao, S.; Gao, J.; Zhou, Y.; Liu, S.; Ma, C.; Zhao, Y. Fluoride ions detection in aqueous media by unprecedented ring opening of fluorescein dye: A novel multimodal sensor for fluoride ions and its utilization in live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122001.
- Zhou, L.; Chen, Y.; Shao, B.; Cheng, J.; Li, X. Recent advances of small-molecule fluorescent probes for detecting biological hydrogen sulfide. Front. Chem. Sci. Eng. 2022, 16, 34–63.
- Strianese, M.; Brenna, S.; Ardizzoia, G.A.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-pyridine-based zinc (ii) complexes as fluorescent hydrogen sulfide probes. Dalton Trans. 2021, 50, 17075–17085.
- Rabha, M.; Sen, B.; Sheet, S.K.; Aguan, K.; Khatua, S. Cyclometalated iridium (iii) complex of a 1, 2, 3-triazole-based ligand for highly selective sensing of pyrophosphate ion. Dalton Trans. 2022, 51, 11372–11380.
- Lai, Q.; Liu, Q.; He, Y.; Zhao, K.; Wei, C.; Wojtas, L.; Shi, X.; Song, Z. Triazole-imidazole (TA-IM) derivatives as ultrafast fluorescent probes for selective Ag+ detection. Org. Biomol. Chem. 2018, 16, 7801–7805.
- Li, G.; Gao, G.; Cheng, J.; Chen, X.; Zhao, Y.; Ye, Y. Two new reversible naphthalimide-based fluorescent chemosensors for Hg2+. Luminescence 2016, 31, 992–996.
- Gopalakrishnan, A.K.; Angamaly, S.A.; Pradeep, S.D.; Madhusoodhanan, D.T.; Manoharan, D.K.; Mohanan, P.V. A Novel Imidazole Bound Schiff Base as Highly Selective “Turn-on” Fluorescence Sensor for Zn2+ and Colorimetric Kit for Co2+. J. Fluoresc. 2022, 32, 189–202.
- Pandith, A.; Uddin, N.; Choi, C.H.; Kim, H.-S. Highly selective imidazole-appended 9, 10-N, N′-diaminomethylanthracene fluorescent probe for switch-on Zn2+ detection and switch-off H2PO4− and CN− detection in 80% aqueous DMSO, and applications to sequential logic gate operations. Sens. Actuators B Chem. 2017, 247, 840–849.
- Kırpık, H.; Kose, M.; Ballı, J.N. Tridentate benzimidazole ligand and its metal complexes: Synthesis, characterization, photo physical and sensor properties. Appl. Organomet. Chem. 2020, 34, e5992.
- Mahnashi, M.H.; Mahmoud, A.M.; Alkahtani, S.A.; Ali, R.; El-Wekil, M.M. A novel imidazole derived colorimetric and fluorometric chemosensor for bifunctional detection of copper (II) and sulphide ions in environmental water samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117846.
- Pan, J.; Yu, J.; Qiu, S.; Zhu, A.; Liu, Y.; Ban, X.; Li, W.; Yu, H.; Li, L. A novel dibenzimidazole-based fluorescent probe with high sensitivity and selectivity for copper ions. J. Photochem. Photobiol. A Chem. 2021, 406, 113018.
- Yin, Y.; Zhang, S.; He, X.; Xu, X.; Zhang, G.; Yang, L.; Kong, L.; Yang, J. A novel tetraphenylethylene-functionalized arylimidazole AIEgen for detections of picric acid and Cu2+. Chem. Pap. 2021, 75, 6297–6306.
- Shi, Y.; Chen, X.; Mi, Z.; Zheng, R.; Fan, J.; Gu, Q.; Zhang, Y. A New Tetrasubstituted Imidazole Based Difunctional Probe for UV-spectrophotometric and Fluorometric Detecting of Fe 3+ Ion in Aqueous Solution. Chem. Res. Chin. Univ. 2019, 35, 200–208.
- Daly, M.; Sutin, A.R.; Robinson, E. Depression reported by US adults in 2017–2018 and March and April 2020. J. Affect. Disord. 2021, 278, 131–135.
- Sahu, B.; Bhatia, R.; Kaur, D.; Choudhary, D.; Rawat, R.; Sharma, S.; Kumar, B. Design, synthesis and biological evaluation of oxadiazole clubbed piperazine derivatives as potential antidepressant agents. Bioorganic Chem. 2023, 136, 106544.
- Kumar, B.; Prakash Gupta, V.; Kumar, V. A perspective on monoamine oxidase enzyme as drug target: Challenges and opportunities. Curr. Drug Targets 2017, 18, 87–97.
- Kumar, B.; Mantha, A.K.; Kumar, V. Recent developments on the structure–activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683.
- Priyadarshini, R.; Raj, G.M. Histamine, Serotonin, Bradykinin, and the Ergot Alkaloids. In Introduction to Basics of Pharmacology and Toxicology: Volume 2: Essentials of Systemic Pharmacology: From Principles to Practice; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2, p. 283.
- Paul, A.; Anandabaskar, N.; Mathaiyan, J.; Raj, G.M. Introduction to Basics of Pharmacology and Toxicology: Volume 2: Essentials of Systemic Pharmacology: From Principles to Practice; Springer Nature: Berlin/Heidelberg, Germany, 2021.
- Charvériat, M.; Guiard, B.P. Serotonergic neurons in the treatment of mood disorders: The dialogue with astrocytes. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 259, pp. 197–228.
- Park, J.; Jeong, W.; Yun, C.; Kim, H.; Oh, C.-M. Serotonergic Regulation of Hepatic Energy Metabolism. Endocrinol. Metab. 2021, 36, 1151.
- Shah, P.A.; Park, C.J.; Shaughnessy, M.P.; Cowles, R.A. Serotonin as a mitogen in the gastrointestinal tract: Revisiting a familiar molecule in a new role. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1093–1104.
- Kanova, M.; Kohout, P. Serotonin—Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837.
- Zweckstetter, M.; Dityatev, A.; Ponimaskin, E. Structure of serotonin receptors: Molecular underpinning of receptor activation and modulation. Signal Transduct. Target. Ther. 2021, 6, 243.
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473.
- Healy, D. Serotonin and depression. BMJ 2015, 350, h1771.
- Murphy, S.E.; Capitão, L.P.; Giles, S.L.; Cowen, P.J.; Stringaris, A.; Harmer, C.J. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: Efficacy, predictors, and mechanisms of action. Lancet Psychiatry 2021, 8, 824–835.
- Zoega, H.; Kieler, H.; Nørgaard, M.; Furu, K.; Valdimarsdottir, U.; Brandt, L.; Haglund, B. Use of SSRI and SNRI antidepressants during pregnancy: A population-based study from Denmark, Iceland, Norway and Sweden. PLoS ONE 2015, 10, e0144474.
- Stahl, M.S.; Lee-Zimmerman, C.; Cartwright, S.; Ann Morrissette, D. Serotonergic drugs for depression and beyond. Curr. Drug Targets 2013, 14, 578–585.
- Zheng, G.; Xue, W.; Wang, P.; Yang, F.; Li, B.; Li, X.; Li, Y.; Yao, X.; Zhu, F. Exploring the inhibitory mechanism of approved selective norepinephrine reuptake inhibitors and reboxetine enantiomers by molecular dynamics study. Sci. Rep. 2016, 6, 1–13.
- Lovinger, D.M. Communication networks in the brain: Neurons, receptors, neurotransmitters, and alcohol. Alcohol Res. Health 2008, 31, 196–214.
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641.
- He, J.H.; Liu, R.P.; Peng, Y.M.; Guo, Q.; Zhu, L.B.; Lian, Y.Z.; Hu, B.L.; Fan, H.H.; Zhang, X.; Zhu, J.H. Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation. J. Neuroinflamm. 2021, 18, 47.
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438.
- Singh, K.; Bhatia, R.; Kumar, B.; Singh, G.; Monga, V. Design strategies, chemistry and therapeutic insights of multi-target directed ligands as antidepressant agents. Curr. Neuropharmacol. 2022, 20, 1329–1358.
- Kerru, N.; Bhaskaruni, S.V.; Gummidi, L.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Synth. Commun. 2019, 49, 2437–2459.
- Naowarojna, N.; Cheng, R.; Lopez, J.; Wong, C.; Qiao, L.; Liu, P. Chemical modifications of proteins and their applications in metalloenzyme studies. Synth. Syst. Biotechnol. 2021, 6, 32–49.
- Kaur, R.; Kumar, B.; Dwivedi, A.R.; Kumar, V. Regioselective alkylation of 1, 2, 4-triazole using ionic liquids under microwave conditions. Green Process. Synth. 2016, 5, 233–237.
- Thenrajan, T.; Sankar, S.S.; Kundu, S.; Wilson, J. Bimetallic nickel iron zeolitic imidazolate fibers as biosensing platform for neurotransmitter serotonin. Colloid Polym. Sci. 2022, 300, 223–232.
- Kamijo, S.; Yamamoto, Y. Recent progress in the catalytic synthesis of imidazoles. Chem. Asian J. 2007, 2, 568–578.
- Marzouk, A.A.; Abu-Dief, A.M.; Abdelhamid, A.A. Hydrothermal preparation and characterization of ZnFe2O4 magnetic nanoparticles as an efficient heterogeneous catalyst for the synthesis of multi-substituted imidazoles and study of their anti-inflammatory activity. Appl. Organomet. Chem. 2018, 32, e3794.
- Nejatianfar, M.; Akhlaghinia, B.; Jahanshahi, R. Cu (II) immobilized on guanidinated epibromohydrin-functionalized γ-Fe2O3@ TiO2 (γ-Fe2O3@ TiO2-EG-Cu (II)): A highly efficient magnetically separable heterogeneous nanocatalyst for one-pot synthesis of highly substituted imidazoles. Appl. Organomet. Chem. 2018, 32, e4095.
- Eidi, E.; Kassaee, M.Z.; Nasresfahani, Z. Synthesis of 2, 4, 5-trisubstituted imidazoles over reusable CoFe2O4 nanoparticles: An efficient and green sonochemical process. Appl. Organomet. Chem. 2016, 30, 561–565.
- Maleki, A.; Alrezvani, Z.; Maleki, S. Design, preparation and characterization of urea-functionalized Fe3O4/SiO2 magnetic nanocatalyst and application for the one-pot multicomponent synthesis of substituted imidazole derivatives. Catal. Commun. 2015, 69, 29–33.
- Kazemi, M. Reusable nanomagnetic catalysts in synthesis of imidazole scaffolds. Synth. Commun. 2020, 50, 2095–2113.
- Negi, A.; Mirallai, S.I.; Konda, S.; Murphy, P.V. An improved method for synthesis of non-symmetric triarylpyridines. Tetrahedron 2022, 121, 132930.
- Bertrand, J.; Dostálová, H.; Kryštof, V.; Jorda, R.; Delgado, T.; Castro-Alvarez, A.; Mella, J.; Cabezas, D.; Faúndez, M.; Espinosa-Bustos, C. Design, Synthesis, In Silico Studies and Inhibitory Activity towards Bcr-Abl, BTK and FLT3-ITD of New 2, 6, 9-Trisubstituted Purine Derivatives as Potential Agents for the Treatment of Leukaemia. Pharmaceutics 2022, 14, 1294.
- Georgiou, M.; Lougiakis, N.; Tenta, R.; Gioti, K.; Baritaki, S.; Gkaralea, L.-E.; Deligianni, E.; Marakos, P.; Pouli, N.; Stellas, D. Discovery of New 1, 4, 6-Trisubstituted-1 H-pyrazolo pyridines with Anti-Tumor Efficacy in Mouse Model of Breast Cancer. Pharmaceutics 2023, 15, 787.
- Li, X.; Naeem, A.; Xiao, S.; Hu, L.; Zhang, J.; Zheng, Q. Safety challenges and application strategies for the use of dendrimers in medicine. Pharmaceutics 2022, 14, 1292.
- Skwarecki, A.S.; Nowak, M.G.; Milewska, M.J. Synthetic strategies in construction of organic low molecular-weight carrier-drug conjugates. Bioorganic Chem. 2020, 104, 104311.
- Girish, Y.R.; Kumar, K.S.S.; Thimmaiah, K.N.; Rangappa, K.S.; Shashikanth, S. ZrO2-β-cyclodextrin catalyzed synthesis of 2, 4, 5-trisubstituted imidazoles and 1, 2-disubstituted benzimidazoles under solvent free conditions and evaluation of their antibacterial study. RSC Adv. 2015, 5, 75533–75546.
- Bajpai, S.; Singh, S.; Srivastava, V. Nano zirconia catalysed one-pot synthesis of some novel substituted imidazoles under solvent-free conditions. RSC Adv. 2015, 5, 28163–28170.
- Fang, S.; Yu, H.; Yang, X.; Li, J.; Shao, L. Nickel-Catalyzed Construction of 2, 4-Disubstituted Imidazoles via C–C Coupling and C− N Condensation Cascade Reactions. Adv. Synth. Catal. 2019, 361, 3312–3317.
- Shi, S.; Xu, K.; Jiang, C.; Ding, Z. ZnCl2-Catalyzed Cycloaddition of Benzimidates and 2 H-Azirines for the Synthesis of Imidazoles. J. Org. Chem. 2018, 83, 14791–14796.
- Man, L.; Copley, R.C.; Handlon, A.L. Thermal and photochemical annulation of vinyl azides to 2-aminoimidazoles. Org. Biomol. Chem. 2019, 17, 6566–6569.
- Harisha, M.B.; Dhanalakshmi, P.; Suresh, R.; Kumar, R.R.; Muthusubramanian, S.; Bhuvanesh, N. TMSOTf-Catalysed Synthesis of 2, 4, 5-Trisubstituted Imidazoles from Vinyl Azides and Nitriles. ChemistrySelect 2019, 4, 2954–2958.
- Tian, Y.; Qin, M.; Yang, X.; Zhang, X.; Liu, Y.; Guo, X.; Chen, B. Acid-catalyzed synthesis of imidazole derivatives via N-phenylbenzimidamides and sulfoxonium ylides cyclization. Tetrahedron 2019, 75, 2817–2823.
