Brown adipose tissue (BAT) plays an important role in energy homeostasis by generating heat from chemical energy via uncoupled oxidative phosphorylation. Besides its high mitochondrial content and its exclusive expression of the uncoupling protein 1, another key feature of BAT is the high expression of myoglobin (MB), a heme-containing protein that typically binds oxygen, thereby facilitating the diffusion of the gas from cell membranes to mitochondria of muscle cells. In addition, MB also modulates nitric oxide (NO•) pools and can bind C16 and C18 fatty acids, which indicates a role in lipid metabolism. Recent sStudies in humans and mice implicated MB present in BAT in the regulation of lipid droplet morphology and fatty acid shuttling and composition, as well as mitochondrial oxidative metabolism.
- brown adipose tissue
- myoglobin
- mitochondrial oxidative metabolism
- energy metabolism
1. Introduction
2. Structure, Location, and Classical Functions of MB
2.1. Structure
The monomeric MB protein in of mice and men, in its mature state (after the removal of the initial methionine), is comprised of a single polypeptide chain of 153 amino acids and has a size of 17 kDa [4]. The globin superfamily, including hemoglobin, myoglobin, cytoglobin, neuroglobin, and globin X, carries out a variety of functions related to the ability of their prosthetic heme group to bind diatomic gaseous ligands [22]. Typical of members of this superfamily, the MB fold consists of a series of eight alpha helices that are tightly wrapped around the heme group. The central iron ion of the heme prosthetic group has six coordination sites. Four sites are bound to nitrogen atoms of the porphyrin ring, and the fifth is bound to the proximal histidine residue (His 93) of the globin protein [23]. Gaseous ligands such as oxygen (O2), nitric oxide (NO•), and carbon monoxide (CO) reversibly bind at the sixth coordinate site of the ferrous heme iron (Fe2+), where the distal histidine (His 64) of the globin facilitates the gas binding through hydrogen bonding [24].2.2. Location
MB is typically known as the heme-binding globin in the cytoplasm of cardiac and skeletal myocytes. However, MB expression is not exclusive to myocytic cells, as it was later reported to also occur as a protein in the liver, brain, and gills of hypoxia-tolerant common carp [25]. Moreover, a distinct MB transcript has been detected in human and murine brain tissues, which differs from the previously seen neuroglobin, a member of the hemoprotein superfamily expressed in neural tissues [25][26]. In common carp, Fraser et al. showed comparable MB levels in the liver and muscle tissues [25], while Cossins et al. reported a discrepant MB expression pattern in different tissues of common carp and zebrafish, such as the liver, brain, kidney, gill, intestine, and eye [27]. With the strongest expression in the heart, the MB expression pattern in humans seems different relative to carp [27][28]. Human MB RNA levels were 333 times and 25 times higher in cardiac muscle than in healthy colon and breast tissues, respectively [28]. In carp and zebrafish, MB protein levels in the liver, gill, and brain comprised less than 1% of the heart levels [27]. Hence, MB is expressed in different lineages, but how the protein’s function might depend on its abundance level and/or expression site is of fundamental relevance for textbooks to come. Much evidence has been accumulated for the muscle-independent expression of MB in cancerous tissues, suggesting a functional role of MB in malignant tissues [29][30][31][32][33][34][35][36][37][38][39][40][41]. Depending on the cancer context, MB can have a positive (tumor-suppressing) [29][30][37][39][40][42][43] or negative (tumor-promoting) [31][41] impact on patients’ survival. Spanning from alleged interplay with the tumor suppressor p53 to impacting mitochondrial respiration, crosstalk with hormonal receptors, and cell survival and/or death implications, MB might interfere with tumor metabolism. Thorough characterization is warranted to decipher the molecular function(s) of the endogenously over-expressed MB by addressing its exact bio-molecular mechanism(s) and the protein‘s interactions with other cancer hallmarks in tumor cells. These analyses should help to determine whether MB itself will turn out to be one new independent cancer hallmark one day.2.3. Classic Gas-Binding Functions
In terrestrial mammals, MB occurs in high concentrations of ~350–700 μM, while in breath-holding deep-diving specialists (whales and large seals), its intracellular abundance can climb even to low millimolar concentrations. These high concentrations are required to sustain muscle contraction via aerobic metabolism. At millimolar levels, MB can temporarily store O2 during the submergence of mammalian apnea divers. MB might also buffer short phases of exercise-induced increases in O2 flux by supplying the gas to the respiring mitochondria of myocytes of terrestrial mammals [5][6][7]. As MB’s O2 affinity exceeds that of hemoglobin, the myocytic globin can acquire O2 from hemoglobin and transport it from the cell membrane into mitochondria for energy production. While progressive MB desaturation is observed during hypoxia or exercise, the physiological significance of MB-derived O2 in supporting mitochondrial oxidative metabolism remains uncertain. MB can only bind a single O2 molecule in contrast to hemoglobin. Whether MB-derived O2 can significantly aid in the amplified oxidative combustion of fuels in working muscles has been questioned from a stoichiometric perspective. However, the protein’s high expression level in myocytes might put such skepticism to rest. While the loss of systemic MB expression (MB-knockout or MBko) did not alter the exercise capacities of mice [44], the resulting cardiac and vascular compensations supported the globin’s O2 supply role in vivo [6]. However, other studies showed these MBko mice to encounter faster fatigue and run shorter distances on a treadmill [45] or revealed no genotypic differences in a wheel-running paradigm [21]. Beyond O2 binding, MB has also been reported to scavenge/detoxify reactive oxygen species (ROS) [46] as well as to maintain NO• homeostasis in cardiomyocytes by either scavenging (during normoxia, MB as MBO2) or producing it (during low O2 environments (hypoxia), MB as deoxy-MB) [47][48]. Similar to deoxygenated hemoglobin (deoxyhemoglobin) [49][50], which turns blood-borne nitrite into NO• to facilitate vasodilation, deoxy-MB exhibits nitrite reductase activity by converting ferrous (Fe2+) myoglobin into metmyoglobin (Fe3+) while NO• is generated (Formula (1)). Notably, nitrite reduction by deoxy MB occurs approximately 36 times faster than that by deoxyhemoglobin due to its low heme redox potential [51].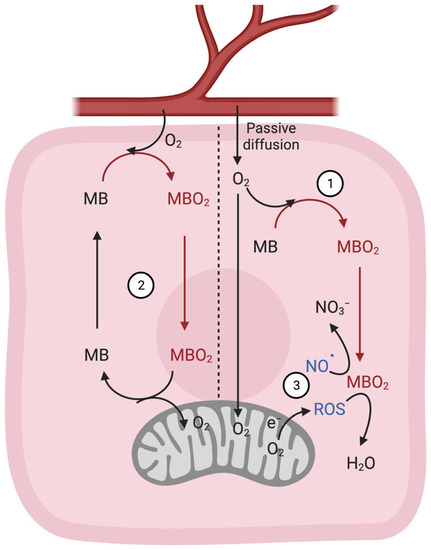
3. Fatty Acid Homeostasis in BAT Thermogenesis and Novel Roles of MB in Lipid Metabolism
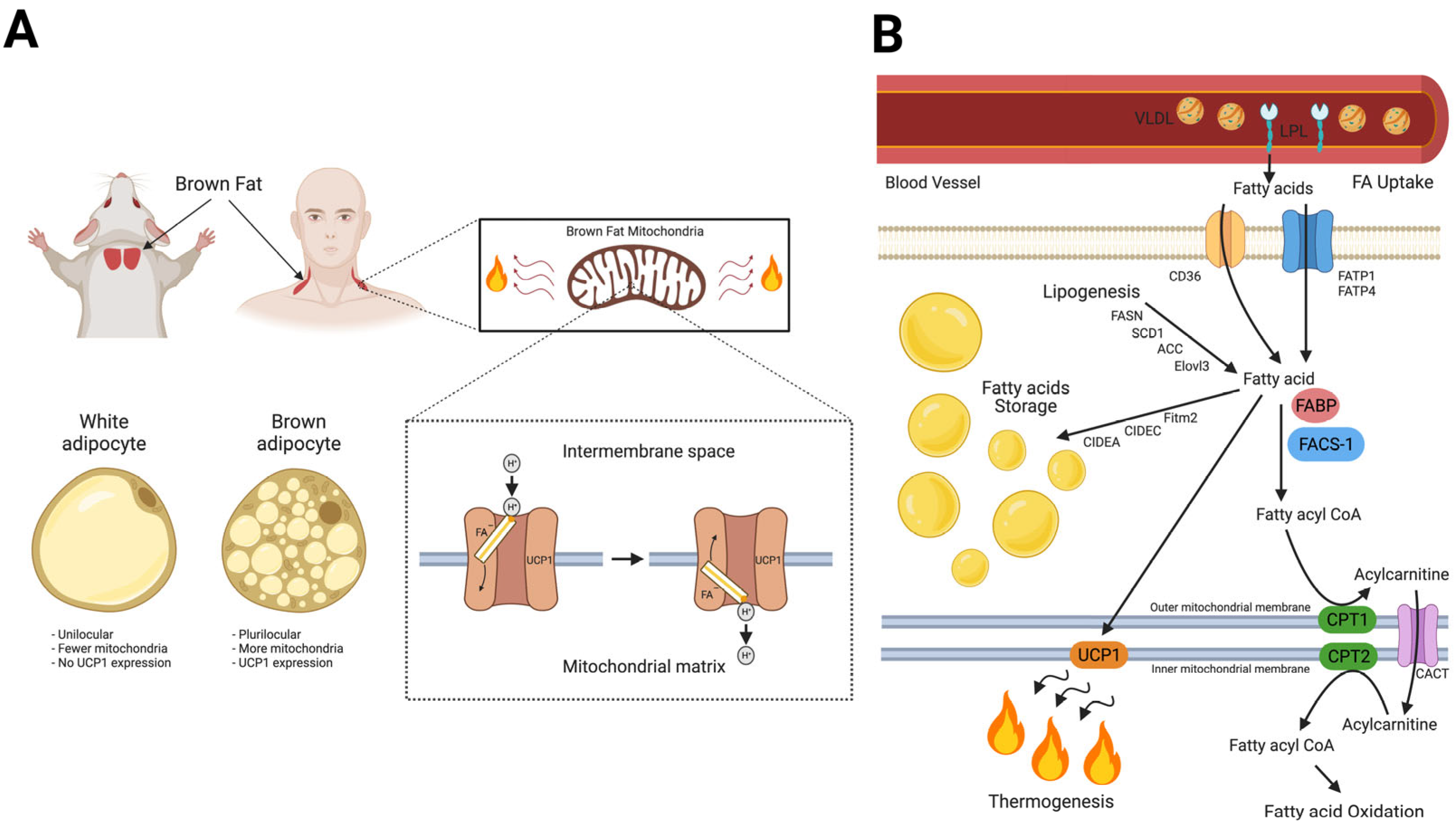
4. Myoglobin in BAT; State-of-the-Art Research
4.1. Expression
4.2. Regulation of Mitochondrial Metabolism and UCP1 Expression
4.3. Regulation of Lipid Metabolism
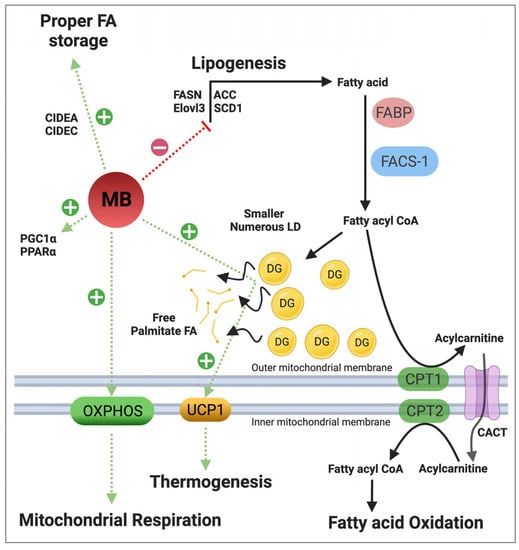
4.4. Regulation of NO• Metabolism
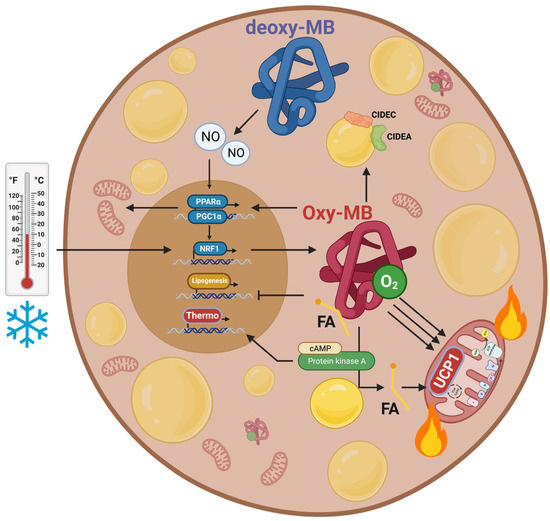
4.5. Regulation of Energy Expenditure and Clinical Implications
References
- Lankester, E.R. Ueber das Vorkommen von Haemoglobin in den Muskeln der Mollusken und die Verbreitung desselben in den lebendigen Organismen. Arch. Gesamte Physiol. Menschen Tiere 1871, 4, 315–320.
- Günther, H. Über den Muskelfarbstoff. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1921, 230, 146–178.
- Kendrew, J.C.; Dickerson, R.E.; Strandberg, B.E.; Hart, R.G.; Davies, D.R.; Phillips, D.C.; Shore, V.C. Structure of myoglobin: A three-dimensional Fourier synthesis at 2 A. resolution. Nature 1960, 185, 422–427.
- Kendrew, J.C.; Bodo, G.; Dintzis, H.M.; Parrish, R.G.; Wyckoff, H.; Phillips, D.C. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature 1958, 181, 662–666.
- Endeward, V.; Gros, G.; Jürgens, K.D. Significance of myoglobin as an oxygen store and oxygen transporter in the intermittently perfused human heart: A model study. Cardiovasc. Res. 2010, 87, 22–29.
- Gödecke, A.; Flögel, U.; Zanger, K.; Ding, Z.; Hirchenhain, J.; Decking, U.K.; Schrader, J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc. Natl. Acad. Sci. USA 1999, 96, 10495–10500.
- Grange, R.W.; Meeson, A.; Chin, E.; Lau, K.S.; Stull, J.T.; Shelton, J.M.; Williams, R.S.; Garry, D.J. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am. J. Physiol.-Cell Physiol. 2001, 281, C1487–C1494.
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222.
- Din, M.U.; Saari, T.; Raiko, J.; Kudomi, N.; Maurer, S.F.; Lahesmaa, M.; Fromme, T.; Amri, E.Z.; Klingenspor, M.; Solin, O.; et al. Postprandial Oxidative Metabolism of Human Brown Fat Indicates Thermogenesis. Cell Metab. 2018, 28, 207–216.
- Forner, F.; Kumar, C.; Luber, C.A.; Fromme, T.; Klingenspor, M.; Mann, M. Proteome Differences between Brown and White Fat Mitochondria Reveal Specialized Metabolic Functions. Cell Metab. 2009, 10, 324–335.
- Timmons, J.A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 2007, 104, 4401–4406.
- Aboouf, M.A.; Armbruster, J.; Thiersch, M.; Gassmann, M.; Goedecke, A.; Gnaiger, E.; Kristiansen, G.; Bicker, A.; Hankeln, T.; Zhu, H.; et al. Myoglobin, expressed in brown adipose tissue of mice, regulates the content and activity of mitochondria and lipid droplets. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 159026.
- Armbruster, J.; Aboouf, M.A.; Gassmann, M.; Egert, A.; Schorle, H.; Hornung, V.; Schmidt, T.; Schmid-Burgk, J.L.; Kristiansen, G.; Bicker, A.; et al. Myoglobin regulates fatty acid trafficking and lipid metabolism in mammary epithelial cells. PLoS ONE 2022, 17, e0275725.
- Chintapalli, S.V.; Jayanthi, S.; Mallipeddi, P.L.; Gundampati, R.; Kumar, T.K.S.; van Rossum, D.B.; Anishkin, A.; Adams, S.H. Novel Molecular Interactions of Acylcarnitines and Fatty Acids with Myoglobin. J. Biol. Chem. 2016, 291, 251133–251143.
- Hendgen-Cotta, U.B.; Esfeld, S.; Coman, C.; Ahrends, R.; Klein-Hitpass, L.; Flögel, U.; Rassaf, T.; Totzeck, M. A novel physiological role for cardiac myoglobin in lipid metabolism. Sci. Rep. 2017, 7, 43219.
- Schlater, A.E.; De Miranda, M.A.; Frye, M.A.; Trumble, S.J.; Kanatous, S.B. Changing the paradigm for myoglobin: A novel link between lipids and myoglobin. J. Appl. Physiol. 2014, 117, 307–315.
- Schlater, A.E.; De Miranda, M.A.; Corley, A.M.; Kanatous, S.B. Lipid stimulates myoglobin expression in skeletal muscle cells. FASEB J. 2012, 26, 1078.16.
- Blackburn, M.L.; Wankhade, U.D.; Ono-Moore, K.D.; Chintapalli, S.V.; Fox, R.; Rutkowsky, J.M.; Willis, B.J.; Tolentino, T.; Lloyd, K.C.K.; Adams, S.H. On the potential role of globins in brown adipose tissue: A novel conceptual model and studies in myoglobin knockout mice. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E47–E62.
- Christen, L.; Broghammer, H.; Rapohn, I.; Mohlis, K.; Strehlau, C.; Ribas-Latre, A.; Gebhardt, C.; Roth, L.; Krause, K.; Landgraf, K.; et al. Myoglobin-mediated lipid shuttling increases adrenergic activation of brown and white adipocyte metabolism and is as a marker of thermogenic adipocytes in humans. Clin. Transl. Med. 2022, 12, e1108.
- Gotz, F.M.; Hertel, M.; Groschelstewart, U. Fatty-Acid-Binding of Myoglobin Depends on Its Oxygenation. Biol. Chem. Hoppe-Seyler 1994, 375, 387–392.
- Jue, T.; Simond, G.; Wright, T.J.; Shih, L.F.; Chung, Y.R.; Sriram, R.; Kreutzer, U.; Davis, R.W. Effect of fatty acid interaction on myoglobin oxygen affinity and triglyceride metabolism. J. Physiol. Biochem. 2016, 73, 359–370.
- Gorr, T.A.; Wichmann, D.; Pilarsky, C.; Theurillat, J.P.; Fabrizius, A.; Laufs, T.; Bauer, T.; Koslowski, M.; Horn, S.; Burmester, T.; et al. Old proteins–new locations: Myoglobin, haemoglobin, neuroglobin and cytoglobin in solid tumours and cancer cells. Acta Physiol. 2011, 202, 563–581.
- Nelson, D.L.; Cox, M.M.; Lehninger, A.L. Lehninger Principles of Biochemistry, 7th ed.; W.H. Freeman and Company: New York, NY, USA; Basingstoke, UK, 2017; I45p.
- Springer, B.A.; Egeberg, K.D.; Sligar, S.G.; Rohlfs, R.J.; Mathews, A.J.; Olson, J.S. Discrimination between oxygen and carbon monoxide and inhibition of autooxidation by myoglobin. Site-directed mutagenesis of the distal histidine. J. Biol. Chem. 1989, 264, 3057–3060.
- Fraser, J.; de Mello, L.V.; Ward, D.; Rees, H.H.; Williams, D.R.; Fang, Y.; Brass, A.; Gracey, A.Y.; Cossins, A.R. Hypoxia-inducible myoglobin expression in nonmuscle tissues. Proc. Natl. Acad. Sci. USA 2006, 103, 2977–2981.
- Burmester, T.; Weich, B.; Reinhardt, S.; Hankeln, T. A vertebrate globin expressed in the brain. Nature 2000, 407, 520–523.
- Cossins, A.R.; Williams, D.R.; Foulkes, N.S.; Berenbrink, M.; Kipar, A. Diverse cell-specific expression of myoglobin isoforms in brain, kidney, gill and liver of the hypoxia-tolerant carp and zebrafish. J. Exp. Biol. 2009, 212 Pt 5, 627–638.
- Bicker, A.; Dietrich, D.; Gleixner, E.; Kristiansen, G.; Gorr, T.A.; Hankeln, T. Extensive transcriptional complexity during hypoxia-regulated expression of the myoglobin gene in cancer. Hum. Mol. Genet. 2014, 23, 479–490.
- Aboouf, M.A.; Armbruster, J.; Guscetti, F.; Thiersch, M.; Gassmann, M.; Gorr, T.A. The role of myoglobin in breast cancer. Cancer Res. 2021, 81, PS19-17.
- Aboouf, M.A.; Armbruster, J.; Thiersch, M.; Guscetti, F.; Kristiansen, G.; Schraml, P.; Bicker, A.; Petry, R.; Hankeln, T.; Gassmann, M.; et al. Pro-Apoptotic and Anti-Invasive Properties Underscore the Tumor-Suppressing Impact of Myoglobin on a Subset of Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 11483.
- Behnes, C.L.; Bedke, J.; Schneider, S.; Kuffer, S.; Strauss, A.; Bremmer, F.; Strobel, P.; Radzun, H.J. Myoglobin expression in renal cell carcinoma is regulated by hypoxia. Exp. Mol. Pathol. 2013, 95, 307–312.
- Bicker, A.; Nauth, T.; Gerst, D.; Aboouf, M.A.; Fandrey, J.; Kristiansen, G.; Gorr, T.A.; Hankeln, T. The role of myoglobin in epithelial cancers: Insights from transcriptomics. Int. J. Mol. Med. 2020, 45, 385–400.
- Brooks, J.J. Immunohistochemistry of soft tissue tumors. Myoglobin as a tumor marker for rhabdomyosarcoma. Cancer 1982, 50, 1757–1763.
- Emoto, M.; Iwasaki, H.; Kikuchi, M.; Shirakawa, K. Characteristics of cloned cells of mixed mullerian tumor of the human uterus. Carcinoma cells showing myogenic differentiation in vitro. Cancer 1993, 71, 3065–3075.
- Eusebi, V.; Bondi, A.; Rosai, J. Immunohistochemical localization of myoglobin in nonmuscular cells. Am. J. Surg. Pathol. 1984, 8, 51–55.
- Flonta, S.E.; Arena, S.; Pisacane, A.; Michieli, P.; Bardelli, A. Expression and functional regulation of myoglobin in epithelial cancers. Am. J. Pathol. 2009, 175, 201–206.
- Kristiansen, G.; Rose, M.; Geisler, C.; Fritzsche, F.R.; Gerhardt, J.; Luke, C.; Ladhoff, A.M.; Knuchel, R.; Dietel, M.; Moch, H.; et al. Endogenous myoglobin in human breast cancer is a hallmark of luminal cancer phenotype. Br. J. Cancer 2010, 102, 1736–1745.
- Lamovec, J.; Zidar, A.; Bracko, M.; Golouh, R. Primary bone sarcoma with rhabdomyosarcomatous component. Pathol. Res. Pract. 1994, 190, 51–60.
- Meller, S.; Bicker, A.; Montani, M.; Ikenberg, K.; Rostamzadeh, B.; Sailer, V.; Wild, P.; Dietrich, D.; Uhl, B.; Sulser, T.; et al. Myoglobin expression in prostate cancer is correlated to androgen receptor expression and markers of tumor hypoxia. Virchows Arch. 2014, 465, 419–427.
- Meller, S.; Van Ellen, A.; Gevensleben, H.; Bicker, A.; Hankeln, T.; Gorr, T.A.; Sailer, V.; Droge, F.; Schrock, F.; Bootz, F.; et al. Ectopic Myoglobin Expression Is Associated with a Favourable Outcome in Head and Neck Squamous Cell Carcinoma Patients. Anticancer. Res. 2016, 36, 6235–6241.
- Oleksiewicz, U.; Daskoulidou, N.; Liloglou, T.; Tasopoulou, K.; Bryan, J.; Gosney, J.R.; Field, J.K.; Xinarianos, G. Neuroglobin and myoglobin in non-small cell lung cancer: Expression, regulation and prognosis. Lung Cancer 2011, 74, 411–418.
- Kristiansen, G.; Hu, J.M.; Wichmann, D.; Stiehl, D.P.; Rose, M.; Gerhardt, J.; Bohnert, A.; ten Haaf, A.; Moch, H.; Raleigh, J.; et al. Endogenous Myoglobin in Breast Cancer Is Hypoxia-inducible by Alternative Transcription and Functions to Impair Mitochondrial Activity. A role in tumor suppression? J. Biol. Chem. 2011, 286, 43417–43428.
- Aboouf, M.A.; Armbruster, J.; Guscetti, F.; Thiersch, M.; Boss, A.; Gödecke, A.; Winning, S.; Padberg, C.; Fandrey, J.; Kristiansen, G.; et al. Endogenous myoglobin expression in mouse models of mammary carcinoma reduces hypoxia and metastasis in PyMT mice. Sci. Rep. 2023, 13, 7530.
- Garry, D.J.; Ordway, G.A.; Lorenz, L.N.; Radford, N.B.; Chin, E.R.; Grange, R.W.; Bassel-Duby, R.; Williams, R.S. Mice without myoglobin. Nature 1998, 395, 905–908.
- Merx, M.W.; Gödecke, A.; Flögel, U.; Schrader, J. Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. FASEB J. 2005, 19, 1015–1017.
- Flogel, U.; Godecke, A.; Klotz, L.O.; Schrader, J. Role of myoglobin in the antioxidant defense of the heart. FASEB J. 2004, 18, 1156–1158.
- Flogel, U.; Merx, M.W.; Godecke, A.; Decking, U.K.; Schrader, J. Myoglobin: A scavenger of bioactive NO. Proc. Natl. Acad. Sci. USA 2001, 98, 735–740.
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261.
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505.
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647.
- Huang, Z.; Shiva, S.; Kim-Shapiro, D.B.; Patel, R.P.; Ringwood, L.A.; Irby, C.E.; Huang, K.T.; Ho, C.; Hogg, N.; Schechter, A.N.; et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Investig. 2005, 115, 2099–2107.
- Brunori, M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem. Sci. 2001, 26, 21–23.
- Brunori, M. Myoglobin strikes back. Protein Sci. 2010, 19, 195–201.
- Shiva, S.; Brookes, P.S.; Patel, R.P.; Anderson, P.G.; Darley-Usmar, V.M. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2001, 98, 7212–7217.
- Beaudry, J.L.; Kaur, K.D.; Varin, E.M.; Baggio, L.L.; Cao, X.; Mulvihill, E.E.; Stem, J.H.; Campbell, J.E.; Scherer, P.E.; Drucker, D.J. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol. Metab. 2019, 22, 37–48.
- Beaudry, J.L.; Kaur, K.D.; Varin, E.M.; Baggio, L.L.; Cao, X.M.; Mulvihill, E.E.; Bates, H.E.; Campbell, J.E.; Drucker, D.J. Physiological roles of the GIP receptor in murine brown adipose tissue. Mol. Metab. 2019, 28, 14–25.
- Kobilka, B.K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 794–807.
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204.
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Saraf, M.K.; Annamalai, P.; Yfanti, C.; Chao, T.; Wong, D.; Shinoda, K.; et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 2016, 23, 1200–1206.
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205.
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359.
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106.
- Golozoubova, V.; Hohtola, E.; Matthias, A.; Jacobsson, A.; Cannon, B.; Nedergaard, J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001, 15, 2048–2050.
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209.
- Divakaruni, A.S.; Humphrey, D.M.; Brand, M.D. Fatty acids change the conformation of uncoupling protein 1 (UCP1). J. Biol. Chem. 2012, 287, 36845–36853.
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413.
- Bertholet, A.M.; Kirichok, Y. The Mechanism FA-Dependent H(+) Transport by UCP1. Handb. Exp. Pharmacol. 2019, 251, 143–159.
- Davis, T.R.; Johnston, D.R.; Bell, F.C.; Cremer, B.J. Regulation of shivering and non-shivering heat production during acclimation of rats. Am. J. Physiol. 1960, 198, 471–475.
- Depocas, F.; Hart, J.S.; Heroux, O. Cold acclimation and the electromyogram of unanesthetized rats. J. Appl. Physiol. 1956, 9, 404–408.
- Frontini, A.; Cinti, S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010, 11, 253–256.
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517.
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerback, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525.
- Zingaretti, M.C.; Crosta, F.; Vitali, A.; Guerrieri, M.; Frontini, A.; Cannon, B.; Nedergaard, J.; Cinti, S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009, 23, 3113–3120.
- Cinti, S. Between brown and white: Novel aspects of adipocyte differentiation. Ann. Med. 2011, 43, 104–115.
- Kozak, L.P.; Anunciado-Koza, R. UCP1: Its involvement and utility in obesity. Int. J. Obes. 2008, 32 (Suppl. S7), S32–S38.
- Nicholls, D.G.; Rial, E. A history of the first uncoupling protein, UCP1. J. Bioenerg. Biomembr. 1999, 31, 399–406.
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118.
- Available online: http://biogps.org/ (accessed on 20 July 2023).
- Fournier, B.; Murray, B.; Gutzwiller, S.; Marcaletti, S.; Marcellin, D.; Bergling, S.; Brachat, S.; Persohn, E.; Pierrel, E.; Bombard, F.; et al. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol. Cell Biol. 2012, 32, 2871–2879.
- Watanabe, M.; Yamamoto, T.; Kakuhata, R.; Okada, N.; Kajimoto, K.; Yamazaki, N.; Kataoka, M.; Baba, Y.; Tamaki, T.; Shinohara, Y. Synchronized changes in transcript levels of genes activating cold exposure-induced thermogenesis in brown adipose tissue of experimental animals. Biochim. Biophys. Acta 2008, 1777, 104–112.
- Klein, J.; Fasshauer, M.; Klein, H.H.; Benito, M.; Kahn, C.R. Novel adipocyte lines from brown fat: A model system for the study of differentiation, energy metabolism, and insulin action. Bioessays 2002, 24, 382–388.
- Sveidahl Johansen, O.; Ma, T.; Hansen, J.B.; Markussen, L.K.; Schreiber, R.; Reverte-Salisa, L.; Dong, H.; Christensen, D.P.; Sun, W.; Gnad, T.; et al. Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell 2021, 184, 3502–3518.e33.
- Gnad, T.; Scheibler, S.; von Kugelgen, I.; Scheele, C.; Kilic, A.; Glode, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399.
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801.
- Bartelt, A.; Widenmaier, S.B.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgul, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 292–303.
- Wittenberg, B.A. Both hypoxia and work are required to enhance expression of myoglobin in skeletal muscle. Focus on “Hypoxia reprograms calcium signaling and regulates myoglobin expression”. Am. J. Physiol.-Cell Physiol. 2009, 296, C390–C392.
- Koma, R.; Shibaguchi, T.; Perez Lopez, C.; Oka, T.; Jue, T.; Takakura, H.; Masuda, K. Localization of myoglobin in mitochondria: Implication in regulation of mitochondrial respiration in rat skeletal muscle. Physiol. Rep. 2021, 9, e14769.
- Yamada, T.; Takakura, H.; Jue, T.; Hashimoto, T.; Ishizawa, R.; Furuichi, Y.; Kato, Y.; Iwanaka, N.; Masuda, K. Myoglobin and the regulation of mitochondrial respiratory chain complex IV. J. Physiol. 2016, 594, 483–495.
- Wu, Z.D.; Puigserver, P.; Andersson, U.; Zhang, C.Y.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124.
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839.
- Wicksteed, B.; Dickson, L.M. PKA Differentially Regulates Adipose Depots to Control Energy Expenditure. Endocrinology 2017, 158, 464–466.
- Balkow, A.; Jagow, J.; Haas, B.; Siegel, F.; Kilic, A.; Pfeifer, A. A novel crosstalk between Alk7 and cGMP signaling differentially regulates brown adipocyte function. Mol. Metab. 2015, 4, 576–583.
- Mitschke, M.M.; Hoffmann, L.S.; Gnad, T.; Scholz, D.; Kruithoff, K.; Mayer, P.; Haas, B.; Sassmann, A.; Pfeifer, A.; Kilic, A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013, 27, 1621–1630.
- Nisoli, E.; Clementi, E.; Tonello, C.; Sciorati, C.; Briscini, L.; Carruba, M.O. Effects of nitric oxide on proliferation and differentiation of rat brown adipocytes in primary cultures. Br. J. Pharmacol. 1998, 125, 888–894.
- Ceddia, R.P.; Collins, S. A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin. Sci. 2020, 134, 473–512.
- Movafagh, S.; Crook, S.; Vo, K. Regulation of Hypoxia-Inducible Factor-1a by Reactive Oxygen Species: New Developments in an Old Debate. J. Cell Biochem. 2015, 116, 696–703.
- Carraway, M.S.; Suliman, H.B.; Jones, W.S.; Chen, C.W.; Babiker, A.; Piantadosi, C.A. Erythropoietin Activates Mitochondrial Biogenesis and Couples Red Cell Mass to Mitochondrial Mass in the Heart. Circ. Res. 2010, 106, 1722-U1111.
- Aboouf, M.A.; Guscetti, F.; von Buren, N.; Armbruster, J.; Ademi, H.; Ruetten, M.; Melendez-Rodriguez, F.; Rulicke, T.; Seymer, A.; Jacobs, R.A.; et al. Erythropoietin receptor regulates tumor mitochondrial biogenesis through iNOS and pAKT. Front. Oncol. 2022, 12, 976961.
- Nisoli, E.; Falcone, S.; Tonello, C.; Cozzi, V.; Palomba, L.; Fiorani, M.; Pisconti, A.; Brunelli, S.; Cardile, A.; Francolini, M.; et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. USA 2004, 101, 16507–16512.
- Szelenyi, Z. Effect of cold exposure on oxygen tension in brown adipose tissue in the non-cold adapted adult rat. Acta Physiol. Acad. Sci. Hung. 1968, 33, 311–316.
- Xue, Y.; Petrovic, N.; Cao, R.; Larsson, O.; Lim, S.; Chen, S.; Feldmann, H.M.; Liang, Z.; Zhu, Z.; Nedergaard, J.; et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009, 9, 99–109.
- Saha, S.K.; Kuroshima, A. Nitric oxide and thermogenic function of brown adipose tissue in rats. Jpn. J. Physiol. 2000, 50, 337–342.
- Kikuchi-Utsumi, K.; Gao, B.H.; Ohinata, H.; Hashimoto, M.; Yamamoto, N.; Kuroshima, A. Enhanced gene expression of endothelial nitric oxide synthase in brown adipose tissue during cold exposure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R623–R626.
- Stone, J.R.; Marletta, M.A. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry 1996, 35, 1093–1099.
- Sebag, S.C.; Zhang, Z.Y.; Qian, Q.W.; Li, M.; Zhu, Z.Y.; Harata, M.; Li, W.X.; Zingman, L.V.; Liu, L.M.; Lira, V.A.; et al. ADH5-mediated NO bioactivity maintains metabolic homeostasis in brown adipose tissue. Cell Rep. 2021, 37, 110003.
- Wu, C.; Orozco, C.; Boyer, J.; Leglise, M.; Goodale, J.; Batalov, S.; Hodge, C.L.; Haase, J.; Janes, J.; Huss, J.W.; et al. BioGPS: An extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009, 10, R130.
- Nagashima, T.; Ohinata, H.; Kuroshima, A. Involvement of Nitric-Oxide in Noradrenaline-Induced Increase in Blood-Flow through Brown Adipose-Tissue. Life Sci. 1994, 54, 17–25.
- Ono-Moore, K.D.; Olfert, I.M.; Rutkowsky, J.M.; Chintapalli, S.V.; Willis, B.J.; Blackburn, M.L.; Williams, D.K.; O’Reilly, J.; Tolentino, T.; Lloyd, K.C.K.; et al. Metabolic physiology and skeletal muscle phenotypes in male and female myoglobin knockout mice. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E63–E79.
