Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marco Durante and Version 2 by Lindsay Dong.
Carbon-ion radiotherapy is a potential elective treatment option for hypoxic tumors. Its high linear energy transfer enables enhanced cell killing in radiation-resistant tumors, while the Bragg peak ensures precise targeting. Clinical evidence in pancreatic and cervical cancers supports positive outcomes of carbon treatments. However, the power of carbon ions against tumor hypoxia is generally underexploited and should be considered to improve the clinical benefit.
- hypoxia
- carbon ions
- particle therapy
- radiotherapy
1. Introduction
In the field of cancer treatment, carbon-ion beams are usually considered an attractive option for cases in which conventional radiotherapy approaches have limitations, offering improved tumor control while preserving patients’ quality of life. According to the statistics of the Particle Therapy Cooperation Group [1], there are 14 centers operating in Europe and Asia that provide cancer treatments with accelerated carbon ions (C-ions).
Carbon ions, like protons, exhibit a characteristic dose distribution known as the Bragg peak, depositing most of their energy at the tumor site, thereby minimizing damage to critical organs beyond the target area [2][4]. When compared to light ions, they have a smaller lateral dose penumbra at greater depths, which makes treatment plans more conformal than in proton therapy [3][5]. However, the main benefit of C-ion radiotherapy (CIRT) lies in its superior biological effectiveness, which is attributed to the densely ionizing nature of C ions in the Bragg peak region [4][6]. Ionization density is described by linear energy transfer (LET), which is proportional to z2/β2 [2][4] and is therefore especially high for heavy, slow ions. High-LET radiation induces a higher fraction of direct DNA damage compared to X-rays, where most of the damage is caused by free radicals produced in water, and the lesions are more difficult to repair [4][6]. From the treatment planning point of view, this enhanced cell kill leads to an increase in the peak-to-plateau ratio in the spread-out Bragg peak (SOBP, a region of a uniform dose in the target volume) and allows for an increase in biological tumor dose without causing additional normal tissue toxicity. Among the drawbacks of C ions is the dose ‘tail’ in the healthy tissue beyond the SOBP that is generated by the projectile fragments having a similar velocity and direction but a longer range [2][4].
2. Tumor Hypoxia and Radioresistance
Hypoxia [5][13] is a characteristic feature of solid tumors associated with their disrupted and heterogeneous vascular network, which is known to correlate with poor prognosis in cancer patients. Briefly, tumors develop their vascular system via angiogenesis, utilizing the blood supply from the host organ. However, in tumors, there is no appropriate balance between pro- and antiangiogenic signals [6][14], and the tumor neovasculature is rather chaotic, dilated, and leaky, sharing both the regular and chaotic features of venules, arterioles, and capillaries [7][15]. Thus, it can no longer meet the metabolic demands of the developing tumor, leading to the formation of oxygen-deficient regions [8][16]. There is no strict and universal hypoxia threshold for all tumor types; however, at partial oxygen pressure (pO2) levels below approximately 35 mmHg, physiological activities and functions have been suggested to become progressively restricted [9][17]. Lack of oxygen can have a significant impact on the production of circulating tumor cells (CTCs) and on the metastatic capacity of cancer cells [10][18]. Hypoxia-induced epithelial–mesenchymal transition contributes to CTC generation, promoting migratory and invasive properties [11][12][19,20]. Hypoxia triggers the activation of hypoxia-inducible factors (HIFs), including HIF-1, which regulates the expression of dozens of genes and mediates pathways influencing metabolism, angiogenesis, cell growth and differentiation, survival, and apoptosis [13][14][23,24]. HIFs are found to be elevated in various cancer types [15][25], making them important targets for pharmacological intervention [16][26]. Two types of hypoxia are usually distinguished: chronic and acute. Chronic, or diffusion-limited hypoxia, caused by limitations in oxygen diffusion from tumor microvessels was first suggested based on histological data from bronchus carcinoma patients [17][27]. Regardless of its type (although there is evidence of acute hypoxia having a stronger impact [18][32]), hypoxia affects radiotherapy outcomes due to the crucial role of oxygen in the biological effectiveness of radiation. Already in the 1950s, it was demonstrated that tissues with increased oxygenation are more radiosensitive than hypoxic tissues [19][33]. In radiotherapy, hypoxia-induced radioresistance is typically described by the oxygen enhancement ratio (OER), i.e., the ratio between the radiation dose in hypoxia and the radiation dose in fully oxygenated conditions (air) resulting in the same biological effect. In conventional radiotherapy, OER can reach a maximum value of 3 [20][36]. The decrease in OER observed with increasing levels of pO2 in tissue is characterized by a sigmoid curve [21][37].3. CIRT for Hypoxic Tumors: Evidence of Effectiveness
3.1. Decrease in OER with Increasing Particle LET: Mechanisms, In Vitro Data, and In Silico Studies
Multiple approaches have been proposed to mitigate hypoxia-induced radioresistance in recent decades, including approaches involving agents that either increase oxygen delivery or radiosensitize or preferentially kill the hypoxic cells, physics-based approaches such as dose painting by boosting the radiation dose to the hypoxic areas, and the use of high-LET heavy ions. As previously noted, heavy ions produce damage predominantly through direct interaction with the biological targets and are therefore less dependent on free radical production and surrounding oxygen concentrations [22][38]. The track effects and production of reactive oxygen species modulate HIF expression [23][24][43,44], which is reduced following C-ion irradiation compared to X-ray irradiation [25][26][45,46]. Carbon ions seem to be able to induce equal cell killing in chronic and acute hypoxia, while in anoxia, the cells are more resistant in acute conditions [27][28][47,48]. Experiments with high-LET neutrons and alpha particles showed a decrease in OER compared to X-rays already many years ago. A full OER–LET relationship was originally measured at the LBNL [29][49] and later confirmed at NIRS (now QST) in Japan [30][50]. The measured OER values ranged from 3 (photons) to 1 for heavy ions at LET > 300 keV/μm in cells irradiated with 12C or 20Ne ions.3.2. Carbon Radiation for Hypoxic Tumors: Preclinical Studies
Pioneering studies conducted at the LBNL were the first to measure OER after exposure to heavy ions [31][32][58,59] in vivo. The authors compared the efficacy of Si, Ne, and C ions in the treatment of rat rhabdomyosarcoma tumors, showing that OER was reduced with increasing beam LET in animals in hypoxia, which was induced by nitrogen gassing of the animal. A few decades later, a study employing a xenograft model of human non-small cell lung cancer further demonstrated substantial differences between X-ray and C-ion radiation; the latter induced a ninefold reduction in HIF-1α levels and significantly delayed tumor growth [33][60]. Studies in Japan [34][35][61,62] included mice with squamous cell carcinoma cells injected into the hind limbs, with hypoxia induced by limb clamping. In vivo–in vitro colony assays of the extracted irradiated tumor cells further validated the reduced OER values of samples irradiated with carbon in comparison to those exposed to X-rays.3.3. Benefits of CIRT for Hypoxic Tumors: Clinical Evidence
Despite the increasing in vitro and in vivo evidence supporting the potential of high-LET radiation to tackle hypoxia, the clinical evidence that C ions radiation increases tumor control probability (TCP) in hypoxic tumors remains scarce. Positive results have been obtained in two highly hypoxic types of tumor: pancreas adenocarcinoma and uterine cervical cancer. Furthermore, the use of carbon ions is being considered for glioblastomas.3.3.1. Pancreatic Cancer
Characteristic features of pancreatic cancer include the immunosuppressive tumor microenvironment [36][67] and significantly higher hypoxia levels than most solid tumors [37][38][68,69], contributing to increased invasiveness and therapy resistance. A unique feature of pancreatic tumor histology is the extensive desmoplasia around the tumor [39][70], which is the formation of dense fibrotic tissue, leading, among consequences, to an increase in interstitial pressure and compression of blood vessels.3.3.2. Cervical Cancer
Uterine cervical cancer is another example of hypoxic cancer that can be efficiently treated with C ions. Highly hypoxic in both primary and especially recurrent forms [40][80], these tumors exhibit poor control rates compared to oxygenated cases [41][81]. The outcomes of the clinical CIRT treatments in 1995–2000 at NIRS in Japan, during which pO2 was measured with an oxygen electrode in individual patients, show comparable disease-free survival and local control rates between hypoxic and oxygenated tumors, indicating the reduction in hypoxia-induced tumor radioresistance with C-ions [42][82]. This was the first clinical attempt to directly demonstrate the ability of LET beams to decrease OER and successfully control hypoxic tumors. More recent findings from Japan’s working group on gynecological tumors [43][44][83,84] further demonstrate favorable 5- and 10-year local control rates for cervical carcinomas treated with carbon beams.3.3.3. Glioblastoma
Glioblastoma (GBM), the most common type of brain tumor [45][85], remains one of the most resistant cancer types. With the standard of care being surgical resection followed by adjuvant radiotherapy with temozolomide, the median survival time of newly diagnosed patients is only 15 months [46][86]. While GBM resistance is explained primarily by infiltrative growth in surrounding brain tissue, it is further impacted by significant levels of hypoxia with pO2 values below 10 mmHg [47][87]. In this regard, C-ions are seen as a solution to escalate the GBM dose while minimizing normal brain tissue damage [48][49][50][88,89,90].4. Tumor Reoxygenation and Local Oxygenation Changes
One of the main rationales for fractionation in radiotherapy is tumor reoxygenation [51][95], i.e., supplying oxygen to the surviving previously hypoxic tumor regions (Figure 12), which, in certain conditions, can outweigh the effects of sublethal injury repair and regeneration.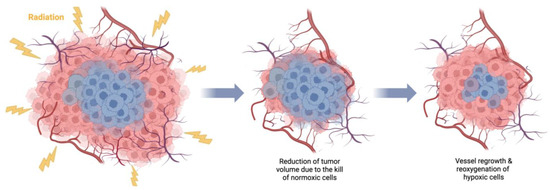
Figure 12. Tumor reoxygenation following irradiation. Death of irradiated normoxic (red color) cells causes tumor shrinkage, which, in turn, leads to the regrowth of blood vessels and supply of previously hypoxic (blue color) cells with oxygen, increasing their radiosensitivity. Created with BioRender.com.
Two types of oxygenation have been described [52][54]: slow reoxygenation of chronically hypoxic cells resulting from tumor shrinkage and changes in acute hypoxia between treatment fractions. The success of carbon treatments for hypoxic tumors can be partly attributed to the careful selection of fractionation schemes, enabling local oxygenation changes to occur between fractions.
It is interesting to note that early studies at NIRS showed accelerated reoxygenation after C-ion compared to X-ray irradiation. In particular, a mouse tumor study [53][101] compared reoxygenation in squamous cell carcinoma and mammary sarcoma in the hind limbs after priming irradiation with either C-ion SOBP or X-rays; the authors observed faster reoxygenation after the carbon treatment for two out of three tested tumors.
5. Is Carbon LET High Enough?
In treatment planning, several beams of different energies are used to produce an SOBP. Especially for large tumors, this unavoidably leads to a “dilution” of the LET values, which remain high for the single Bragg peak but with a much lower mean value. The highest values of dose-averaged LET (LETd) are typically reached around the planning tumor volume (PTV) edges, remaining at low to medium levels in the core, where hypoxia often occurs, leading to inferior clinical outcomes in larger tumors. Large margins around the gross tumor volume (GTV) leads to a relatively low LET distribution exactly where it is most needed, i.e., in the hypoxic subvolumes of the GTV. Furthermore, equal target dose coverage can be achieved, with plans resulting in very different LET distributions [54][105]. For C ions, a common range of the LETd in the tumor core is 30–80 keV/µm.
A clear correlation between the C-ion LETd distribution and tumor control has been demonstrated in pancreatic cancer [55][106], chondrosarcoma [56][107], and sacral chordoma [57][108] patients. In those trials, local recurrence was correlated with low LETd values, suggesting that LETd maps can be used to classify patients into groups at high or low risk of recurrences in high-dose regions [58][109].
6. Strategies to Maximize Carbon-Ion LET and Their Limitations
6.1. Simultaneous Integrated Boost
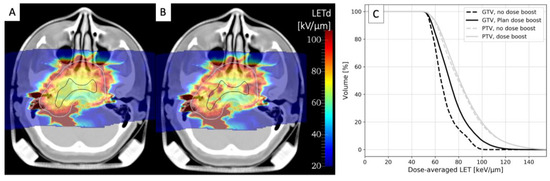
The only approach currently used in clinical trials that might improve the LETd distribution in C-ion treatments is simultaneously integrated boost (SIB). While the main rationale for this approach based on intensity-modulated radiotherapy (IMRT) with X-rays is to deliver a dose boost to selected target subregions during irradiation, it also shifts the LETd distribution to higher values. Figure 24 compares the LETd distributions for a chordoma treatment plan optimized (A) for a uniform PTV dose or (B) for a plan including GTV SIB. As shown by LETd–volume histograms (C), the LETd distribution in the GTV is significantly improved, while almost no changes are visible in the PTV. SIB is already a standard approach in IMRT and is under testing with CIRT in three ongoing clinical trials: for head and neck adenoid cystic carcinoma in at CNAO [59][60][111,112] and for prostate [61][62][113,114] and pancreatic [63][115] tumors at SPHIC.
Figure 24. LETd distribution calculated in 12C two-field treatment plan for chordoma optimized for either a uniform PTV dose of 3 Gy (RBE) (A) or for an additional GTV dose boost of 1.5 Gy (RBE) (B). White and black contours represent PTV and GTV, respectively. (C) LETd–volume histograms for boosted (solid lines) and non-boosted treatment plans (dashed lines) for tumor GTV (black lines) and PTV (grey lines). Calculations were performed using GSI in-house treatment planning software TRiP98 [64][116]. Anonymized CT data were retrieved from the pilot project repository of GSI, where treatment was performed, according to German law. Informed consent is waived by the ethical committee of the University of Heidelberg because of the anonymized nature of the research plans.
6.2. Arc Therapy
.2. Arc Therapy
Another promising strategy in particle therapy is arc therapy, where beams are delivered from multiple angles using a gantry rotating around the patient. Originally aimed at enhancing dose conformity and plan robustness, arc therapy has the additional benefit of increasing LET values within the tumor [65][117]. With arc therapy, the maximum LET for C ions moves from the tumor edges to its center, making the plan less sensitive to the presence of hypoxia in the tumor core [66][67][56,118].
Arc CIRT requires rotating gantries with high magnetic rigidity compared to proton gantries. Despite the use of novel superconductive technologies, C-ion gantries remain large and expensive. At the moment, only five facilities (one in Germany, two in Japan, and two in South Korea) use C-ion gantries. A potential breakthrough in this field is the possible delivery of radiotherapy to patients in an upright position [68][119]; however, rotation speed, acceleration, angular range, and the tolerance of the patients to these parameters still need to be addressed [69][120].
