Water contamination has become a global crisis, affecting millions of people worldwide and causing diseases and illnesses, including cholera, typhoid, and hepatitis A. Conventional water remediation methods have several challenges, including their inability to remove emerging contaminants and their high cost and environmental impact. Nanomembranes offer a promising solution to these challenges. Nanomembranes are thin, selectively permeable membranes that can remove contaminants from water based on size, charge, and other properties. They offer several advantages over conventional methods, including their ability to remove evolving pollutants, low functioning price, and reduced ecological influence. However, there are numerous limitations linked with the applications of nanomembranes in water remediation, including fouling and scaling, cost-effectiveness, and potential environmental impact.
1. Introduction
Water is a precious resource essential for life, and access to clean water is a basic human right. Unfortunately, water contamination has become a global crisis, affecting the health and well-being of millions of people worldwide. Polluted water can cause a range of diseases and illnesses, including cholera, typhoid, hepatitis A, and dysentery, among others. The World Health Organization (WHO) estimates that contaminated water and poor sanitation are responsible for the deaths of approximately 3.4 million people annually, mostly children under the age of five. Conventional methods of water remediation, such as chemical treatment, flocculation, sedimentation, filtration and disinfection that generally involve chlorination, ozonation, and ultraviolet radiation have been exploited for many years to remove contaminants from water. However, these methods have several challenges, including limitations in removing specific contaminants, high cost, and environmental impact
[1][2][1,2].
Nanomembranes offer a promising solution to the challenges associated with conventional water remediation methods. Nanomembranes are thin, selectively permeable membranes, typically less than 100 nanometers in thickness, that can remove contaminants from water based on size, charge, and other properties. Nanomembranes offer several advantages over conventional methods, including their ability to eradicate emerging contaminants, their low operational costs, and their reduced environmental impact. Nanomembranes can remove incipient contaminants, such as pharmaceuticals and pesticides, with high efficiency. The small pore size of nanomembranes allows them to filter out even the smallest particles, including viruses and bacteria. This makes them highly effective in removing emerging contaminants, which are often present in low concentrations and difficult to remove with conventional methods.
Nanomembranes also have low operational costs. Unlike chemical treatment methods, which require large amounts of chemicals, energy, and equipment, nanomembranes require minimal energy and equipment. They also have low maintenance costs, making them an attractive option for water remediation in resource-limited settings. Nanomembranes have a reduced environmental impact compared to conventional methods. They produce less waste and require fewer chemicals, reducing their impact on aquatic ecosystems and wildlife. They also have a smaller carbon footprint, as they require less energy to operate
[3][4].
In spite of having countless latent advantages, the field of nanomembranes still has several challenges that need to be addressed. There are also several challenges associated with the use of nanomembranes in water remediation
[4][5].
One of the main challenges is fouling and scaling, which can occur when contaminants accumulate on the surface of the membrane, reducing its efficiency over time. The fouling of membranes takes place due to the presence of suspended solids (generally >0.01 microns), whereas scaling occurs because of the dissolved solids especially salts, when exceeded from their solubility. Fouling and scaling can be caused by several factors, including the quality of the feed water, the membrane material, and the operating conditions. Addressing fouling and scaling is critical for maintaining the long-term performance of nanomembranes. Fouling occurs when contaminants accumulate on the surface of the membrane, reducing its efficiency over time. Scaling occurs when mineral deposits accumulate on the surface of the membrane, leading to reduced water flux and enhanced pressure drop. Fouling and scaling can be caused by several factors, including the quality of the feed water, the membrane material, and the operating conditions. Fouling and scaling can be mitigated through several strategies, including cleaning and maintenance of the membrane, optimization of operating conditions, and the use of antifouling coatings. However, these strategies can be costly and time-consuming, and their effectiveness may depend on the specific application and membrane material
[5][6][7][8][9][6,7,8,9,10].
Another challenge associated with the nanomembranes is cost-effectiveness. While nanomembranes have low operational costs, they can be expensive to produce, making them inaccessible in many settings. The cost of nanomembranes is mainly due to the high cost of raw materials, such as polymers and ceramics, and the complex manufacturing process required to produce membranes with nanoscale features
[10][11].
2. Nanomembrane Production Methods
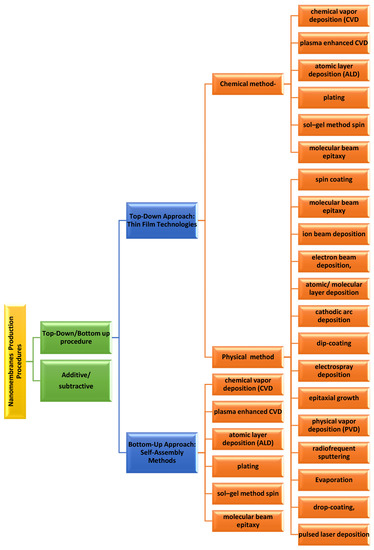
Nanomembrane production procedures encompass two distinct approaches. The first one is the additive/subtractive procedure of manufacturing the nanomembranes and another is the top–down/bottom–up tactic. The additive/subtractive fabrication is the procedure related with the membrane material. As the name indicates, in additive, new material has been incorporated to the membrane, whereas in subtractive, the material is detached from the membrane. The top–down/bottom–up approach explains in what way the addition and subtraction are achieved. Top–down or bottom–up approaches can be of either type, the additive or the subtractive. Details classification is provided in
Figure 1 [11][44].
Figure 1.
Classification of nanomembranes production process.
3. Types of Nanomembranes and Their Water Remediation Applications
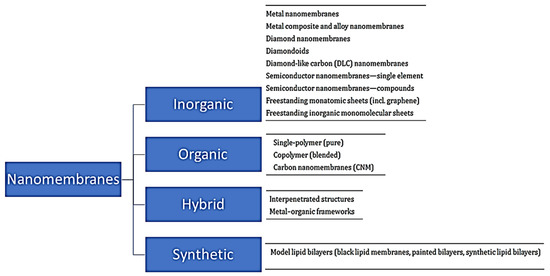
Nanomembranes are broadly classified into inorganic, organic and hybrid types and synthetic biomembrane types (Figure 2) based on the materials they are made from.
4) based on the materials they are made from.
Figure 24. Broad classification of nanomembranes.
3.1. Organic Nanomembranes
Organic nanomembranes are made up entirely of one or more organic constituents that epitomize a significant group of existing self-supporting nanomembranes [12]. Organic nanomembranes are made up entirely of one or more organic constituents that epitomize a significant group of existing self-supporting nanomembranes [45].
There is an enormous number of organic complexes and their blends that could potentially be exploited, although it is important to note that not all organic compounds are suitable for creating nanomembranes. Membranous structures cannot be made from certain organic compounds due to their gaseous or liquid state. Organic materials are composed of carbon compounds, except for pure carbon in either of its allotropic forms, as well as basic carbon compounds like carbides, oxides of carbon, carbonates, and cyanides. Many macromolecular/polymeric structures are well-suited for the production of freestanding nanomembranes, including polysaccharides, synthetic polymers, synthetic lipids, proteins, RNA, and DNA-based membranes [13][14][15][16][17][18]. There is an enormous number of organic complexes and their blends that could potentially be exploited, although it is important to note that not all organic compounds are suitable for creating nanomembranes. Membranous structures cannot be made from certain organic compounds due to their gaseous or liquid state. Organic materials are composed of carbon compounds, except for pure carbon in either of its allotropic forms, as well as basic carbon compounds like carbides, oxides of carbon, carbonates, and cyanides. Many macromolecular/polymeric structures are well-suited for the production of freestanding nanomembranes, including polysaccharides, synthetic polymers, synthetic lipids, proteins, RNA, and DNA-based membranes [46,47,48,49,50,51].
Despite the potential advantages of macromolecular nanomembranes, these materials tend to exhibit a high level of sensitivity to temperature changes, and their beneficial characteristics are typically only maintained within a limited temperature range. They can also be attacked and dissolved by organic solvents and may be endangered by enhanced humidity. Furthermore, their mechanical properties tend to be impaired as membrane thickness reduces, and they typically have a low Young’s modulus. Most macromolecular nanomembranes start to slink and are enduringly plastically distorted underneath permanent pressure. Pyroxylin (nitrocellulose, collodion) was likely the first organic nanomembrane produced, which was created by Bechhold in 1907 [19]. Organic nanomembranes can be further classified into CNM, pure (single polymer) and blended (copolymer type). Despite the potential advantages of macromolecular nanomembranes, these materials tend to exhibit a high level of sensitivity to temperature changes, and their beneficial characteristics are typically only maintained within a limited temperature range. They can also be attacked and dissolved by organic solvents and may be endangered by enhanced humidity. Furthermore, their mechanical properties tend to be impaired as membrane thickness reduces, and they typically have a low Young’s modulus. Most macromolecular nanomembranes start to slink and are enduringly plastically distorted underneath permanent pressure. Pyroxylin (nitrocellulose, collodion) was likely the first organic nanomembrane produced, which was created by Bechhold in 1907 [52]. Organic nanomembranes can be further classified into CNM, pure (single polymer) and blended (copolymer type).
Carbon Nanomembranes
The use of carbonaceous nanomaterials, encompassing graphene, carbon nanotubes (CNTs), carbon nanofibers (CNFs) and fullerenes, has gained widespread popularity in the field of environmental remediation. These nanomaterials possess distinct properties that make them ideal for numerous applications associated with air and water decontamination. Specifically, graphene and its oxide have been utilized as adsorbents for heavy metal ion removal. Furthermore, the occurrence of hydroxyl and carboxyl functional groups present on the graphene oxide surface enhances its sorption capacity. In summary, carbon-based nanomaterials have proved to be effective and versatile tools in the fight against environmental contamination [20][21]. The use of carbonaceous nanomaterials, encompassing graphene, carbon nanotubes (CNTs), carbon nanofibers (CNFs) and fullerenes, has gained widespread popularity in the field of environmental remediation. These nanomaterials possess distinct properties that make them ideal for numerous applications associated with air and water decontamination. Specifically, graphene and its oxide have been utilized as adsorbents for heavy metal ion removal. Furthermore, the occurrence of hydroxyl and carboxyl functional groups present on the graphene oxide surface enhances its sorption capacity. In summary, carbon-based nanomaterials have proved to be effective and versatile tools in the fight against environmental contamination [53,54].
Now, it can be concluded that carbon nanomaterials (CNMs) are versatile objects that can be easily fabricated and adapted to various environments. They possess exceptional durability against heat, electron irradiation, chemical substances, and pressure variations. By altering their precursors and treatment, CNMs can be made conductive or insulating. These materials are exploited in a broad range of applications, including microscopy support, pressure sensor diaphragms, gas and liquid filtration, protective coatings, and in conjunction with other 2D substances. CNMs possess such immense potential due to their 2D structure and effortless surface customization, making them ideal for crafting ultrathin membranes exploited in material separation and filtration. Conventional filtration membranes have a thickness of over 100 nm and operate through either a diffusion–solution process or via narrow pores. These membranes selectively separate a mixture of materials based on varying atomic or molecular species’ diffusion coefficients, resulting in the ideal infusion of specific species. This selectivity allows for the separation of gas or liquid mixtures into their individual constituents.
The diffusion process involved in materials separation through membranes can be slow, requiring high-pressure transformations among the two sides of the membrane to generate sufficient flux (φ). The formula for flux is given as φ = DAΔp/l, where D represents diffusivity, A represents the membrane area, Δp represents the pressure change between the two sides, and l represents the membrane thickness. Thin membranes need lower pressure differences compared to thicker ones to maintain the same level of flux. Two-dimensional membranes such as CNMs and graphene are considered the thinnest membranes and function like “sieves” in materials separation. The molecules can permeate through these membranes in a “ballistic” process, where they either translocate through a pore or bounce off the solid material between the pores. The unique potential of CNMs lies in their two-dimensional geometry and ease of surface modification. The primary application of CNMs is in the production of ultrathin membranes for materials isolation and filtration. To achieve this, the CNMs are altered to suit the specific separation or filtration requirements. Traditional membranes exploited for gas and liquid filtration are relatively thick (>100 nm) and require high-pressure differences to generate a sufficient flux. In contrast, thin CNMs require much lower pressure differences to maintain the same flux due to their “ballistic” permeation process. The emergence of mass-produced two-dimensional membranes may pave the way for CNMs to revolutionize the field of materials separation technology through disruptive innovation.
Pure (single polymer) and blended (copolymer type) nanomembrane:
Nanomembranes of this class consist entirely of organic materials and are typically made up of large macromolecules or polymers such as synthetic lipids, proteins, polysaccharides, RNA, DNA-based membranes, and synthetic polymers. These may be made up of a single polymer and known as pure (single polymer)-based nanomembranes and can be made up of two or more than two different polymer types and called blended (copolymer-type) nanomembranes. The creation of these nanomembranes involves using various types of polymers, including epoxy resins, polysulfone, polycarbonate, polyethersulfone, nylon, polyacrylate, polystyrene, cellulose, nitrocellulose, polyamide, polyimide, polypropylene, polydopamine, polyurethane, polyvinylchloride, poly (methyl methacrylate), polyester, poly(vinylidene fluoride), polytetrafluoroethylene (PTFE, Teflon), poly(lactic acid), polyacrylonitrile and polydimethylsiloxane (PDMS) [22]. Nanomembranes of this class consist entirely of organic materials and are typically made up of large macromolecules or polymers such as synthetic lipids, proteins, polysaccharides, RNA, DNA-based membranes, and synthetic polymers. These may be made up of a single polymer and known as pure (single polymer)-based nanomembranes and can be made up of two or more than two different polymer types and called blended (copolymer-type) nanomembranes. The creation of these nanomembranes involves using various types of polymers, including epoxy resins, polysulfone, polycarbonate, polyethersulfone, nylon, polyacrylate, polystyrene, cellulose, nitrocellulose, polyamide, polyimide, polypropylene, polydopamine, polyurethane, polyvinylchloride, poly (methyl methacrylate), polyester, poly(vinylidene fluoride), polytetrafluoroethylene (PTFE, Teflon), poly(lactic acid), polyacrylonitrile and polydimethylsiloxane (PDMS) [56].
Watanabe and Kunitake (2007) [23] successfully prepared a freestanding epoxy nanomembrane that was 20 nm thick and could be transferred intact onto a wire frame measuring 1 cm in diameter. Although the nanomembrane was too thin to be directly visible from a perpendicular position, light reflection was observed at an angle of 15. The high-quality epoxy nanomembranes did not show any visible defects or cracks. explored the feasibility of employing epoxy resins as a material for nanomembranes, which were subsequently transported onto AAO (anodized aluminum oxide) films for scanning electron microscopy (SEM) analysis. The thinnest freestanding nanomembranes demonstrated a consistent thickness of (23 ± 2) nm, as determined by averaging thickness values obtained from varied locations on the same sampling and dissimilar SEM specimens. These films exhibited flexibility on the uneven contour of the AAO support, which was only observed in films thinner than 40 nm. On the other hand, thicker films (80 and 200 nm) appeared stiff on the SEM support, but all membranes demonstrated flexibility on the macroscopic scale without any observable cracks or defects on the membrane surface. As such, the study concluded that the epoxy membranes were uniform and devoid of defects over their entire area. Watanabe and Kunitake (2007) [57] successfully prepared a freestanding epoxy nanomembrane that was 20 nm thick and could be transferred intact onto a wire frame measuring 1 cm in diameter. Although the nanomembrane was too thin to be directly visible from a perpendicular position, light reflection was observed at an angle of 15. The high-quality epoxy nanomembranes did not show any visible defects or cracks. explored the feasibility of employing epoxy resins as a material for nanomembranes, which were subsequently transported onto AAO (anodized aluminum oxide) films for scanning electron microscopy (SEM) analysis. The thinnest freestanding nanomembranes demonstrated a consistent thickness of (23 ± 2) nm, as determined by averaging thickness values obtained from varied locations on the same sampling and dissimilar SEM specimens. These films exhibited flexibility on the uneven contour of the AAO support, which was only observed in films thinner than 40 nm. On the other hand, thicker films (80 and 200 nm) appeared stiff on the SEM support, but all membranes demonstrated flexibility on the macroscopic scale without any observable cracks or defects on the membrane surface. As such, the study concluded that the epoxy membranes were uniform and devoid of defects over their entire area.
Polysulfone (PSU) is a popular membrane material due to its excellent mechanical, thermal, and chemical stability, making it suitable for manufacturing porous membranes for microfiltration (MF) to nanofiltration (NF). However, its low lipophobic properties make it susceptible to fouling during water purification. To overcome this issue, Ahmadipouya (2020) developed nanofiltration membranes by incorporating a metal–organic framework (MOF) into the polysulfone (PSf) matrix to remove organic dyes like methylene blue (MB), malachite green (MG), methyl red (MR), and methyl orange (MO) from water.
Graphene is a two-dimensional material composed of a monolayer of atoms arranged in a honeycomb sp2
carbon lattice. Graphene boasts remarkable mechanical characteristics, chemical stability, and a significant surface area. Graphene-based membranes demonstrate characteristics akin to ceramic membranes and can be fashioned into films through graphene/graphene oxide fluid phase dispersions, mimicking polymers.
Graphene and/or GO can be functionalized to extend their efficiencies by grafting functional groups onto their surface. GO can attract functional groups and act as a potential adsorbent for removing heavy metal ions and organic contaminants. Fused heterocyclic compounds enriched with nitrogen, such as pyrano-pyrazoles, pyrazolo-pyridines, and pyrazolo-pyrido-pyrimidines, have shown expedient biological characteristics and can be exploited for functionalization. Some multicomponent reactions have been developed for synthesizing these heterocycles.
3.2. Hybrid (Inorganic/Organic) Nanomembranes
4.2. Hybrid (Inorganic/Organic) Nanomembranes
In addition to traditional inorganic carbon-based nanomaterials, transition metal-based nanomaterials and organic nanomaterials, there have been proposals to incorporate other nanomaterials for water remediation purposes. One such example is the use of magnetic halloysite nanotube (MHNT) composites that have been modified with molecularly imprinted polymers (MIPs) to selectively recognize and adsorb 2,4,6-trichlorophenol (TCP) for the remediation of wastewater. It has been suggested that this approach has potential for the development of commercially available products. In this work, the researchers developed a magnetic molecularly imprinted polymer (MMIP) for the selective recognition of 2,4,6-trichlorophenol (TCP) using magnetic halloysite nanotubes particles (MHNTs) as the base. Scientists have produced magnetic halloysite nanotubes (MHNTs) by attaching magnetic nanoparticles to carboxylic acid-functionalized halloysite nanotubes (HNTs−COOH) through a high-temperature reaction of ferric Tri acetylacetonate in 1-methyl-2-pyrrolidone.
García-Torres et al., 2022 [24] developed a straightforward method for creating flexible electronic hybrid materials featuring nanostructured surfaces, using free-standing perforated two dimensional nanomembranes that host regimented 1D metal-based nanostructures. The fabrication process involves depositing alternating layers of perforated poly(lactic acid) (PLA) and poly(3,4-ethylenedioxythiophene), which are then incorporated with copper metallic nanowires (NWs) via electrodeposition. The top PLA layer with nanoperforations is then coated with silver through a transmetallation reaction. This approach combined the conformability and flexibility of the ultrathin, soft polymeric nanomembranes with the excellent electrical properties of metals, making it ideal for use in bio-integrated electronic devices. By tailoring the nanomembrane surface chemistry, this work demonstrated its sensing capabilities toward H García-Torres et al., 2022 [84] developed a straightforward method for creating flexible electronic hybrid materials featuring nanostructured surfaces, using free-standing perforated two dimensional nanomembranes that host regimented 1D metal-based nanostructures. The fabrication process involves depositing alternating layers of perforated poly(lactic acid) (PLA) and poly(3,4-ethylenedioxythiophene), which are then incorporated with copper metallic nanowires (NWs) via electrodeposition. The top PLA layer with nanoperforations is then coated with silver through a transmetallation reaction. This approach combined the conformability and flexibility of the ultrathin, soft polymeric nanomembranes with the excellent electrical properties of metals, making it ideal for use in bio-integrated electronic devices. By tailoring the nanomembrane surface chemistry, this work demonstrated its sensing capabilities toward H2
O2
, with a good linear range (0.35–10 mM) of concentration, limit of detection (7 μm) and sensitivity (120 µA cm−2
mM−1
). The hybrid nanomembranes produced were flexible and conformable, with selectivity toward H2
O2
, good stability and reproducibility, and such characteristics were confirmed by EDX, SEM, XPS, EIS, CV and contact angle analyses.
3.3. Inorganic Nanomembranes
4.3. Inorganic Nanomembranes
Inorganic nanomembranes have become gradually widespread recently for their latent use in water remediation applications. These membranes are typically composed of inorganic substances like metals, metal oxides and ceramics. One of the most promising inorganic nanomembranes for water remediation is graphene oxide. This material possesses excellent mechanical and thermal properties, as well as a high surface area, which allows for the efficient adsorption of contaminants. Other inorganic nanomaterials that have been investigated for water remediation include metal–organic frameworks (MOFs), zeolites, and mesoporous silica. MOFs, for example, are highly porous materials with tunable characteristics that can be exploited for the adsorption and degradation of organic contaminants. Inorganic nanomembranes have also been exploited to remove heavy metals from water.
Shayesteh and his co-workers (2016) [25] described the synthesis and performance evaluation of titania–gamma–alumina multilayer nanomembranes. The rejection ratio of a multilayered membrane in both acidic and alkaline solutions was investigated in a recent study. The support for the nanomembrane was prepared using the slip-casting method, which involved the use of alpha-alumina tubes. A gamma–alumina sub-layer and titania top layer were sequentially coated on the support, and the water flux and permeability of the nanomembrane were characterized. The study also examined the nanomembrane’s ability to reject microorganisms and several ions in a model wastewater at different pH levels. The support was evaluated for treating a model wastewater using a nanomembrane. The permeability of water through the nanomembrane was reduced when pressure in the range of 1–10 bar was applied. However, the permeability became almost constant at higher pressures and enhanced water flux. Rejection tests were conducted on a model wastewater containing ions, and the results indicated that the nanomembrane produced by the support fabricated using the slip-casting method could partially reject ions and successfully separate all microorganisms. Adjusting the pH was found to enhance ion rejection. Shayesteh and his co-workers (2016) [87] described the synthesis and performance evaluation of titania–gamma–alumina multilayer nanomembranes. The rejection ratio of a multilayered membrane in both acidic and alkaline solutions was investigated in a recent study. The support for the nanomembrane was prepared using the slip-casting method, which involved the use of alpha-alumina tubes. A gamma–alumina sub-layer and titania top layer were sequentially coated on the support, and the water flux and permeability of the nanomembrane were characterized. The study also examined the nanomembrane’s ability to reject microorganisms and several ions in a model wastewater at different pH levels. The support was evaluated for treating a model wastewater using a nanomembrane. The permeability of water through the nanomembrane was reduced when pressure in the range of 1–10 bar was applied. However, the permeability became almost constant at higher pressures and enhanced water flux. Rejection tests were conducted on a model wastewater containing ions, and the results indicated that the nanomembrane produced by the support fabricated using the slip-casting method could partially reject ions and successfully separate all microorganisms. Adjusting the pH was found to enhance ion rejection.
Silicon-on-insulator (SOI) is a composite material that has gained widespread use in the semiconductor device manufacturing industry. It consists of a thin crystalline layer of Si, known as the template layer, that is separated from a bulk wafer by a SiO2
film. This technology was developed approximately 15 years ago, and it rapidly gained acceptance in the industry due to its ability to produce a thin Si template layer quickly and reliably. SOI has numerous advantages over bulk Si crystal, particularly in low-power circuit applications exploited in portable electronic devices. The use of a thin Si layer on top of an oxide has significantly improved semiconductor device performance.
TiO2 is another coating material with favorable characteristics, such as nontoxicity, stability, low cost and photocatalytic characteristics, as noted by several authors [26]. Recently, CA/PEG membranes were modified with TiO is another coating material with favorable characteristics, such as nontoxicity, stability, low cost and photocatalytic characteristics, as noted by several authors [100]. Recently, CA/PEG membranes were modified with TiO2 in various loadings and exploited in RO and MD. Shafiq and co-workers (2018) [27] found that CA/PEG membranes with TiO in various loadings and exploited in RO and MD. Shafiq and co-workers (2018) [101] found that CA/PEG membranes with TiO2
loaded with 5, 10, 15, 20, and 25 wt% showed maximum desalination rates of 80, 90, 95.4, 85, and 80%, respectively, these results confirmed that the optimal loading was 15 wt% for maximum desalination. Membranes coated with TiO2
and exposed to UV radiation exhibited enhanced lipophobicity and self-cleaning characteristics. However, too much TiO2 blocked membrane pores and reduced membrane performance. Emami and co-workers (2018) and Stan and co-workers (2019) [28][29] observed that TiO blocked membrane pores and reduced membrane performance. Emami and co-workers (2018) and Stan and co-workers (2019) [102,103] observed that TiO2 NPs-coated membranes had exceptional self-cleaning characteristics under ultraviolet irradiation. Kwak and co-workers (2001) [30] conducted a study that revealed a TFC membrane, modified with TiO NPs-coated membranes had exceptional self-cleaning characteristics under ultraviolet irradiation. Kwak and co-workers (2001) [104] conducted a study that revealed a TFC membrane, modified with TiO2
consisting of organic/inorganic hybrids, was less susceptible to fouling as compared to the pure PA membrane when exploited in RO. Safarpour and co-workers. (2015) established a TFN-RO film using interfacial polymerization and coating with reduced graphene oxide/TiO2. The altered membrane demonstrated improved lipophobicity and anti-fouling characteristics compared to the unmodified membrane [31]. Ren and co-workers (2017) created a TiO . The altered membrane demonstrated improved lipophobicity and anti-fouling characteristics compared to the unmodified membrane [105]. Ren and co-workers (2017) created a TiO2-coated PVDF electrospun nanofiber membrane (ENM) that exhibited high flux (73.4 L/m2h) and salt rejection (99.99%) [32]. -coated PVDF electrospun nanofiber membrane (ENM) that exhibited high flux (73.4 L/m2h) and salt rejection (99.99%) [106].
Zinc oxide (ZnO) nanoparticles (NPs) have gained popularity as an additive due to their low price, high stability (physical, chemical, mechanical, and thermal), high surface area, surface functionalization, and remarkable antimicrobial and anti-corrosive characteristics. In membrane modification, ZnO has been shown to enhance the lipophobicity of blended membranes, which improves permeability and fouling resistance [103]. For example, ZnO NPs were incorporated into a cellulose acetate (CA) membrane via electrospinning to improve its antibacterial property for reverse osmosis (RO) [104].
Zinc oxide (ZnO) nanoparticles (NPs) have gained popularity as an additive due to their low price, high stability (physical, chemical, mechanical, and thermal), high surface area, surface functionalization, and remarkable antimicrobial and anti-corrosive characteristics. In membrane modification, ZnO has been shown to enhance the lipophobicity of blended membranes, which improves permeability and fouling resistance [29]. For example, ZnO NPs were incorporated into a cellulose acetate (CA) membrane via electrospinning to improve its antibacterial property for reverse osmosis (RO) [30].
4.4. Synthetic Biological Nanomembranes
3.4. Synthetic Biological Nanomembranes
Model lipid bilayers are the examples of this class, the synthetic organic nanomembranes, which represent replicas of living nanomembranes [44]. The first model lipid bilayers were successfully synthesized in 1962 [111]. Initially known as “black lipid membranes” or “painted bilayers”, they were created as platforms for studying membrane processes in vitro, aiming to facilitate the analysis of transmembrane mechanisms and ion channel function. Among the early achievements in synthetic ion channels, tetra-substituted β-cyclodextrin was the first fully synthetically produced ion channel reported as early as 1982 [112]. This marked a significant advancement in the field, showcasing the potential to artificially create functional channels that mimic natural ion channels found in biological membranes. Subsequently, research in synthetic lipid bilayers and ion channels has continued to progress, leading to further insights into membrane biophysics and their applications in drug delivery, biosensing, and understanding cellular processes. The study of model lipid bilayers and synthetic ion channels remains a critical area of research in biophysics and nanotechnology.
Model lipid bilayers are the examples of this class, the synthetic organic nanomembranes, which represent replicas of living nanomembranes [11]. The first model lipid bilayers were successfully synthesized in 1962 [33]. Initially known as “black lipid membranes” or “painted bilayers”, they were created as platforms for studying membrane processes in vitro, aiming to facilitate the analysis of transmembrane mechanisms and ion channel function. Among the early achievements in synthetic ion channels, tetra-substituted β-cyclodextrin was the first fully synthetically produced ion channel reported as early as 1982 [34]. This marked a significant advancement in the field, showcasing the potential to artificially create functional channels that mimic natural ion channels found in biological membranes. Subsequently, research in synthetic lipid bilayers and ion channels has continued to progress, leading to further insights into membrane biophysics and their applications in drug delivery, biosensing, and understanding cellular processes. The study of model lipid bilayers and synthetic ion channels remains a critical area of research in biophysics and nanotechnology.
45. Characteristics of Nanomembranes Attributed with Water Purification
4.1. Electrical Properties
5.1. Electrical Properties
Electrical characteristics of nanomembranes were investigated using a potentiostat/galvanostat system, which measured the output leakage current of approximately 90 µA at 0.5 V for a 30 nm thick nanomembrane transferred onto a substrate. The electrical resistivity was calculated to be 0.5 × 10
11 Ωcm. This value was found to be only seven times smaller than the value measured for a PCGF-PEI film directly fabricated onto a substrate (3.79 × 10
11 Ωcm), indicating that the insulating behavior was not lost upon detachment from the substrate. The high insulating character of the nanomembrane suggested a defect-free behavior. The electrical resistivity value of the nanomembrane was essentially the same as that of a conventional bisphenol-A-type epoxy resin (10
10–10
12 Ωcm), which was claimed to be highly compatible with many chemical substances and had been exploited to develop superior functional and structural composites. The electrical properties of the nanomembranes were also found to be highly insulating, indicating a defect-free behavior. The resistivity value of the nanomembrane was comparable to that of a conventional bisphenol-A-type epoxy resin, suggesting that the resistivity value remained essentially the same when the material was prepared as a nanomembrane. These findings have important implications for the development of functional and structural composites using nanomembranes. Conclusions have been made that epoxy resins can be utilized as a material for nanomembranes. The resulting membranes were shown to be uniform, defect-free, and flexible, with a consistent thickness of (23 ± 2) nm. Moreover, the thinnest membrane exhibited a tensile strength that is comparable to conventional thick epoxy resins, while its ultimate elongation was substantially lower. These findings provide valuable insights for the development of new applications for epoxy resins in the field of nanotechnology.
4.2. Adsorption
5.2. Adsorption
Certain nanomaterials (NMs) grounded on nanostructured graphene, metal oxides, carbon nanotubes (CNT), zeolite, porous BN and electrospun nanofibers are capable of serving two functions, namely adsorption and the membrane filtration of heavy metal ions, phosphates and nitrates. These NMs possess active sites and high porosity, making them ideal for adsorbing contaminants. Electrospun nanofiber-based membranes that contain NMs exhibit intriguing characteristics for removing trace quantities of contaminants from water through filtration and adsorption, which is due to their porosity and large surface area. The adsorption of contaminants from an aqueous solution by these materials can occur through chemical binding, physical adsorption (caused by porosity, van der Waals attraction and the large surface area of NMs), or electrostatic attraction
[35][114].
4.3. Photocatalysis
5.3. Photocatalysis
The photocatalytic characteristics of TiO
2 NP-based NEMs are distinctive and include photodegradation and photoinduced super-lipophobicity. These characteristics provide the membrane surface with fouling resistant, antimicrobial and self-cleaning characteristics. The excitation of valence electrons of the photocatalyst occurs under UV light, causing their migration and resulting in the observed effects
[36][115].
4.4. Antimicrobial Activity
5.4. Antimicrobial Activity
The use of Silver NPs as antimicrobial mediators for NEM is widespread because of their highly effective biocidal characteristics. Silver intermingles with biochemical compounds, including cysteine, containing thiol groups (S-H) that contain phosphorus and sulfur. Through the formation of S-Ag or di-sulfide bonds, silver deteriorates the microbial proteins, denatures the DNA, and interjects the electron transport system, leading to its biocidal effect. In addition to silver, other nanoparticles such as Cu, CNT, and graphene have also been utilized for modifying commercial membranes to enhance their biocidal efficiency and application duration. These modified membranes deliver effective water disinfection while maintaining a high flux recovery ratio
[37][116].
4.5. Chlorine Resistance
5.5. Chlorine Resistance
Functional nanomaterials such as zeolite and silica are being researched for use in NF and RO membranes due to their promising characteristics. Incorporating GO, SiO
2, CNT and zeolite nanoparticles (NPs) into the barricade sheet of TFNCM has been shown to enhance the membranes’ resistance to chlorine. MWCNTs, in particular, act as a defensive coating for PA against free chlorine attack. Meanwhile, the improved chlorine resistance of GO-based membranes is principally attributed to the creation of hydrogen bonds between GO and PA that hinder the chlorine’s collaboration with active N-H bonds in PA. Zeolite-based membranes have also been explored for desalination as they are chemically robust and favorable.
56. Challenges with Nanomembrane-Enhanced Water Remediation
Nanomembranes have emerged as a potential solution for wastewater remediation due to their unique characteristics, but there are several challenges that need to be addressed. These challenges include the lack of information about the nanomaterials, their potential adverse effects on human health and the environment, and the need for effective and sustainable wastewater remediation methods. The rapid commercialization of nanomembranes has led to an enhancement in their production globally, but they also face various challenges that must be addressed to realize their full potential. Fouling can reduce filtration efficiency and increase operating costs, necessitating frequent cleaning or replacement. Scaling up nanomembrane production to meet large-scale water treatment demands can be challenging and costly. Developing cost-effective manufacturing methods without compromising performance remains a significant obstacle. They are vulnerable to mechanical and chemical degradation, impacting their stability and lifespan. Ensuring durability and longevity under varying water conditions is crucial for practical applications. Achieving high selectivity for target pollutants while avoiding interference from other compounds can be challenging. Fine-tuning membrane properties to selectively capture specific contaminants requires careful material design and engineering. The use of nanomaterials in water treatment raises concerns about potential environmental and health risks. Meeting stringent regulatory requirements and demonstrating the safety of nanomembrane technology is essential for widespread adoption.
Moreover, the performance of nanomembranes can be influenced by the complex and diverse composition of water sources. Variations in water chemistry may impact membrane stability, fouling rates, and contaminant removal efficiency. Some nanomembrane processes may require significant energy inputs for effective water remediation. Developing energy-efficient systems to minimize operational costs and reduce the environmental footprint is a critical challenge. Developing scalable and reproducible manufacturing techniques for nanomembranes is essential for cost-effective production and large-scale implementation. Integrating nanomembranes into existing water treatment systems and infrastructure can present compatibility challenges and require adaptations to ensure seamless operation.
 Encyclopedia
Encyclopedia
