1. Nutritional Supplementation: Collagen Hydrolysates
With limited treatment options and currently no approved DMDs, the potential role of nutritional factors and supplements to treat OA warrants further investigation. This is especially true for patients with pre-clinical or symptomatic OA, as early intervention is key to stopping OA progression and eventual surgery
[1][34]. Consumers are generally interested and open to supplementation, especially if they demonstrate efficacy in promoting health improvement
[2][3][39,40]. Commonly used and readily available supplements advertised to help manage OA are collagen-based products, such as gelatin and CHs
[4][5][16,41].
2. Collagen
Collagen is the most prevalent protein in animals and constitutes approximately 30% of total body protein
[6][42]. It is a structural protein found in connective tissue and characterized by the repeating motif “glycine (Gly)-X-Y”, where X is often proline (Pro) and Y is hydroxyproline (Hyp)
[6][7][42,43]. Collagen has three α-chains of approximately 1000 amino acids (AAs), each of which coils around the other to form a triple helix structure. Collagen triple helices cross-link together using telopeptides found at the ends to form collagen fibrils. Several fibrils align to form collagen fibres. This cross-linking is highly conserved between collagen types. Currently, 29 types of collagen have been identified, although they can vary in amino acid (AA) sequence, structure, function, and associated distribution in tissues and organs
[7][43]. For example, type 1 collagen is typically found in bone, skin, teeth, and tendons, whereas type II is found in cartilage
[4][16].
3. Collagen Hydrolysates
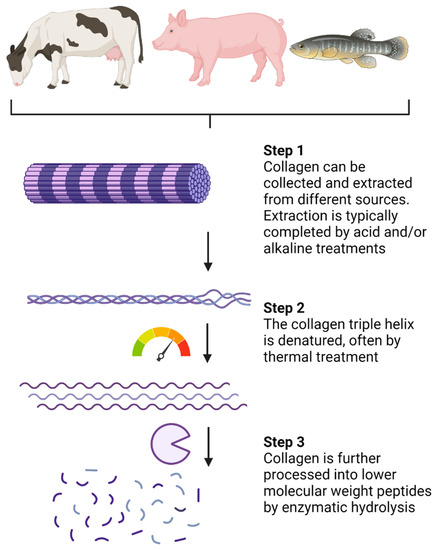
Collagen can be isolated from various sources, including bovine, porcine, and piscine
[4][8][16,44]. Collagen extraction is often a by-product of the meat industry, and the main source of collagen for collagen-based products remains bovine due to its high availability as well as biocompatibility and weak antigenicity
[7][43]. Collagen can be extracted from various tissues such as bones, tendons, and connective tissues
[4][16]. Marine sources of collagen include those mentioned but also skin and scales
[4][16].
Collagen hydrolysates (CHs) are products with low molecular weight (MW) peptides, often between 3 and 6 kDa
[4][16]. CHs are a result of industrialized processed collagen (summarized in
Figure 1) and are sold in the cosmetic, pharmaceutical, and food sectors
[4][16]. Collagen extraction is typically completed by acid and/or alkaline treatments
[4][7][16,43]. Following extraction, thermal treatments, usually above 40 °C, promote denaturation of the collagen chain triple helix (
Figure 1). Enzymatic hydrolysis is then completed to break down peptide chains into lower MW peptides. The choice of processing procedure and proteolytic enzymes can vary between CH products, although pepsin, papain, and Alcalase are often used
[4][16]. Very few CHs are considered ultra-hydrolysates which have a notably lower MW (less than 1 KDa) due to specialized and proprietary processing methods. In comparison, gelatin is also a collagen-based product, but it is obtained through partial hydrolysis of collagen and is, therefore, processed to a lower extent than CHs
[8][44]. The conversion of collagen and gelatin into bioactive products such as CHs makes collagen-sourced products valuable to the pharmaceutical and cosmetic industries.
Figure 1. Processing of native collagen into small low-molecular-weight peptides. Created with Biorender.com.
CHs are nutraceuticals that have multiple applications and are often taken as oral supplements. The health benefits of CHs have been largely ascribed to their bioactive peptide (BAP) content and corresponding sequences
[4][5][8][16,41,44]. The peptide content of CHs is a result of the collagen source and the processing methods described above
[4][16]. Different processing procedures and sources will result in variable peptide sequences and content after extraction and hydrolysis, thereby affecting the overall bioactivity of the CH product. In addition to their BAP content, CHs also contain AAs which contribute to their bioactivity
[4][5][9][16,41,45].
CHs have been demonstrated to provide several health benefits, including antimicrobial and antihypertensive effects, promotion of wound healing and bone synthesis, decreasing joint pain associated with OA, helping in the regulation of inflammation, improving skin health, acting as inhibitors of dipeptidyl peptidase-IV (DPP-IV), in addition to having antioxidant properties and angiotensin-I-converting enzyme inhibitory effects
[4][5][8][16,41,44]. The presence of BAPs, such as Pro-Hyp, alanine (Ala)-Hyp, Pro-Hyp-Gly, and Gly-Pro-Hyp, in the blood after oral consumption of CHs and gelatin has been verified in human clinical studies
[10][11][12][13][46,47,48,49]. In fact, the postprandial absorption of Gly, Pro, and Hyp was significantly greater after the oral consumption of CHs compared to non-enzymatically hydrolyzed collagen, suggesting that processed collagen products have increased absorption and bioavailability
[9][45]. Besides their measurement in plasma, the CH-derived BAPs, Gly-Pro-Hyp, and Pro-Hyp were shown to be excreted in the urine after oral consumption, indicating that these peptides were well absorbed and stable post-absorptively
[14][50]. Clinical studies focusing on the bioactivity of collagen BAPs have helped establish some of their health-promoting properties after oral consumption.
4. Bioactivity and Health Benefits of CHs
4.1. Clinical Studies on CHs and CH-Derived Peptides
The clinical efficacy and safety of CHs and CH-derived peptides have been demonstrated in several trials and summarized in
Table 1 [15][16][17][18][19][20][21][22][23][24][25][51,52,53,54,55,56,57,58,59,60,61]. The supplement primarily helps manage OA pain and increase mobility, but recent work has also demonstrated improvements in bone health and cartilage characteristics, especially in patients engaging in a physical exercise program
[19][23][55,59].
Table 1. Summary of human clinical studies using CHs to treat OA and related joint problems.
| Study Design |
Population |
Supplement Used and Details |
Reference |
| Randomized double-blind, placebo-controlled |
Knee OA |
Colnatur by Ordesa (Eberbach, Germany)
CH with a mean molecular weight of 3500 Da. Sourced from “traceable non-ruminant bones of neutral taste and odour”
Improvement in knee joint pain |
Benito-Ruiz et al., (2009) [15][51] |
| Single-center, prospective, randomized, double-blind, placebo-controlled |
Postmenopausal women with reduced bone mineral density |
FORTIBONE® by Gelita
Described as a mixture of specific bioactive collagen peptides (SCP) with a mean molecular weight of ~5 kDa. However, peptides were not given.
Fortibone is derived from Type I and III bovine collagen
Increased bone mineral density |
König et al., (2018) [16][52] |
| Randomized, double-blind, placebo-controlled |
Knee, hip, elbow, shoulder, hand, and/or lumbar spine OA |
Genacol AminoLock Collagen (MW less than 1kDa)
Source: Bovine collagen. No additional details in the manuscript.
Reduced VAS scores |
Bruyère et al., (2012) [17][53] |
| Randomized, double-blind, placebo-controlled |
Knee OA |
Porcine (supplied by NittaGelatin Inc., Osaka, Japan) and bovine (Nitta Gelatin India Ltd., Panampilly Nagar, India) CH-derived peptides. Peptide sequences not given.
Reduced WOMAC and VAS scores |
Kumar et al., (2015) [18][54] |
| Randomized, double-blind, placebo-controlled study |
Elderly
sarcopenic men |
BODYBALANCE by Gelita using bovine type 1 collagen.
Increased fat-free mass, bone mass, and muscle mass |
Zdzieblik et al., (2015) [19][55] |
Monocentric, prospective, randomized, double-blind,
placebo-controlled |
Athletes with knee pain |
FORTIGEL by Gelita; described as a mixture of collagen peptides. Sequences not given.
Decreased activity-related pain intensity |
Zdzieblik et al., (2017) [20][56] |
| Triple-blind, placebo-controlled, randomized controlled trial |
Knee pain |
Genacol AminoLock Collagen (MW less than 1kDa)
Source: Bovine collagen. No additional details in the manuscript.
Improvement in various joint structures |
Feliciano et al., (2017) [21][57] |
Prospective, randomized,
placebo-controlled, double-blind study |
Athletes with activity-related joint pain |
CH-Alpha from Gelita. No details given.
Diminished joint discomfort and pain |
Clark et al., (2008) [22][58] |
| Single-center, prospective, randomized, placebo-controlled, double-blind, pilot trial |
Mild knee OA |
FORTIGEL by Gelita; described as a mixture of collagen peptides. Sequences not given.
Increased proteoglycan content in knee cartilage and improved cartilage morphology |
McAlindon et al., (2011) [23][59] |
| Randomized double-blind study |
Pre-pubertal Spanish children |
Gelatine Royal (Kraft Foods Europe, Barcelona, Spain), unspecified collagen source.
Improved bone remodelling during growth |
Martin-Bautista et al., (2011) [24][60] |
A randomized double-blind, placebo-controlled study design was completed over 6 months on 250 subjects with primary knee OA to assess the efficacy of CH on OA pain and joint function
[15][51]. Using the visual analogue scale (VAS) to assess for pain, as well as the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index pain subscale, patients showed significant improvement in knee joint pain and comfort following treatment
[15][51]. A similar study recruited 200 patients to participate in a randomized, double-blind, placebo-controlled trial over 6 months to assess the efficacy of CH supplementation on patients with knee OA, but also included patients with hip, elbow, shoulder as well as hand and/or lumbar spine OA
[17][53]. The number of clinical responders, as assessed using VAS, was significantly greater in the CH treatment group compared to the placebo group, and CHs were confirmed to be safe and well tolerated by patients
[17][53]. Significant reductions in WOMAC and VAS scores were also observed in another randomized, double-blind, placebo-controlled clinical study investigating the effectiveness of porcine and bovine CH-derived peptides in patients with knee OA
[18][54]. Although the above study provided an initial indication that the bioactive component of CHs was peptides, they were not sequenced
[18][54]. Instead, an analysis of the AA composition of the porcine and bovine hydrolysates was completed. The composition (g/kg dry weight) was different between the two collagen sources for some AAs. For example, proline was found in greater amounts in the bovine hydrolysate (1.620) compared to porcine (1.550); however, the impact of the AAs profiles on the clinical outcomes was not discussed. The bovine hydrolysate appeared to decrease the WOMAC and VAS scores to a greater extent, but no formal analysis comparing the efficiency of the two hydrolysates was performed.
Recent clinical studies have also demonstrated that collagen peptides alongside resistance training improved body composition. A randomized double-blind placebo-controlled study showed that collagen peptides increased fat-free mass, bone mass, and muscle mass more so than the placebo
[19][55]. The mentioned collagen peptides were part of a commercial product provided by Gelita AG (Eberbach, Germany); however, the sequences and content of the peptides were not provided nor investigated. Instead, the AA composition was assessed (no method given). In a similar study, a triple-blind placebo-controlled randomized controlled trial instructed patients with knee pain to complete a home exercise program together with a treatment of either CH or a placebo
[21][57]. Patients treated with CH showed significant improvement in joint structures, which included decreased cartilage abrasion and lateral meniscus protrusions, as well as a significant increase in cartilage thickening in the central portion of the trochlear articular cartilage
[21][57]. In addition, for the patients who were non-compliant with the home exercise program, VAS scoring still indicated that CH treatment decreased pain
[21][57].
Interestingly, CH supplementation also appears to improve activity-related joint pain, regardless of OA diagnosis. For example, Clark et al. (2008) performed a 24-week clinical study involving 147 healthy athletes with activity-related joint pain who were physically active, fit, and had no evidence of established OA
[22][58]. Joint discomfort and pain in the CH-treated group were significantly reduced. The authors suggested that CHs support joint health and could reduce the risk of further joint deterioration in high-risk groups (e.g., athletes), which merits further investigation. Thus, CHs could act as a preventive treatment and be recommended before potential OA diagnosis. Furthermore, athletes with knee pain also showed improvement in activity-related pain intensity after treatment with collagen BAPs over a 12-week supplementation period
[20][56]. Accordingly, high-risk groups could possibly benefit from supplementation at a young age (e.g., below 25 y) and possibly delay the onset of joint damage, but this remains to be assessed. More recent work by McAlindon et al. (2011) demonstrated that, in a randomized, placebo-controlled, double-blind imaging study, changes in proteoglycan content in knee cartilage, as well as improvement in cartilage morphology, were observed after 24 weeks of CH treatment
[23][59].
CHs have also been shown to improve other articular structures of the joint besides cartilage, most notably in the bone. In a randomized, placebo-controlled double-blind study, König et al. (2018) concluded that bone mineral density (BMD) increased after collagen peptide supplementation compared to placebo
[16][52]. Furthermore, bone markers from plasma also showed significant improvement in bone remodelling homeostasis. Specifically, a biomarker for bone formation, the amino-terminal propeptide of type I collagen, increased in the collagen peptide treatment group, whereas no changes were observed in the placebo group. In contrast, an indicator of bone resorption, the C-telopeptide of type I collagen, increased in the placebo group, with no changes in the collagen treatment group. In summary, plasma biomarkers of bone turnover indicated that collagen peptides increased bone formation, while also decreasing the level of bone resorption.
Another study assessing the effect of CHs in pre-pubertal children concluded that partially hydrolyzed collagen, or in other words, gelatin, could improve bone remodelling during growth and development
[24][60]. Further studies assessing the use of CHs or similar products on young healthy participants are needed, with thorough follow-ups as participants grow and age. This would help determine the preventative potential of CHs in terms of reducing the onset and severity of joint disorders.
There are very few studies that investigate the effects of CHs alone on bone health. Instead, CHs are often used in association with another treatment. For example, the effects of intramuscular injection of calcitonin were compared to calcitonin treatment along with the addition of CHs into the diet in postmenopausal women
[25][26][61,62]. The effects of CHs and calcitonin together increased and prolonged the effects of the calcitonin drug treatment and a greater effect in inhibiting bone collagen breakdown was observed.
Although clinical evidence favouring CH supplementation for joint pain is growing, the mechanisms behind their activity are still not fully established, as well as how CHs compare to other protein hydrolysates. Furthermore, the BAPs profiles of the CHs and the main contributors to bioactivity are rarely investigated. Different processing procedures and sources can affect the peptide content in CHs, which may affect bioactivity. For many commercial CHs, the peptide sequences and technology used to make their respective CHs are protected, trademarked, and often patented. Further transparent evidence-based work is needed to assess the BAPs found in CH products and how they regulate joint health. In that regard, in vitro methods provide these opportunities to investigate the peptide content of CHs and their potential effects.
4.2. In Vitro and Animal Studies on CHs and CH-Derived Peptides
Numerous studies and reviews have detailed the potential bioactivity of collagen products and CH-derived BAPs
[4][5][8][27][16,41,44,63]. The bioactivity of collagen products depends on their source, processing, and bioavailability, i.e., the absorption of the bioactive compounds, such as BAPs and AAs, into the systemic circulation so they may exert their beneficial activity
[4][8][16,44]. CH and CH-derived BAPs have been shown to exhibit antioxidant activity, ACE-inhibitory activity, metal chelating abilities, anti-diabetic properties, antimicrobial potential, and beneficial effects on bone and joint health
[4][7][8][27][28][13,16,43,44,63]. The list of BAPs identified from CHs continues to grow, as well as the associated bioactive functions of the identified peptides. The efforts to create BAP databases have begun
[29][30][31][64,65,66], although these databases remain incomplete. Other databases on proteins and BAPs stemming from CHs and others are also available, and a list of these can be found at
https://biochemia.uwm.edu.pl/bioactive-peptide-databases/ (accessed on 4 August 2023). Some well-established and utilized databases are often updated, maintained, and replenished, although as with other online tools, website maintenance, broken links, offline sites, link removals, and other errors remain an issue when utilizing web-based tools and remain a limiting factor for knowledge access regarding BAPs.
Previous in vitro and in vivo work by Nakatani et al. (2009) has helped establish the chondroprotective effects of porcine CHs, and its main BAP, Pro-Hyp
[27][63]. Using an animal model, C57BL/6J mice were placed on a basic AIN-93G diet, alongside a treatment of excess phosphorus to induce joint damage. Different treatment diets included a negative control (no phosphorus) and control diet (gluten hydrolysate used as a control and excessive phosphorus), a CH treatment group (CH and excessive phosphorus), as well as a peptide group (Pro-Hyp and excessive phosphorus). Mice treated with phosphorus showed joint degradation, notably a decrease in chondrocytes and articular cartilage thickness. As a result of CH and Pro-Hyp treatment, these supplements inhibited the loss of chondrocytes induced by excess phosphorus while also inhibiting cartilage thinning
[27][63]. In the same study, an in vitro model was used where chondrocytes (ATDC5 cells) were treated with Pro, Hyp, Gly, a combination of Pro and Hyp, a mixture of Pro, Hyp and Gly, the single peptides Pro-Hyp and Pro-Hyp-Gly, as well as the same CH used in the above animal study. Key findings from this study showed that CH and Pro-Hyp inhibited chondrocyte mineralization and terminal differentiation, as assessed by alizarin red and ALP staining, respectively. Changes to the ECM components were also observed, notably an increase in glycosaminoglycan, which was determined via alcian blue staining. Using reverse transcription-polymerase chain reaction (RT-PCR), the mRNA content of aggrecan was shown to be increased with Pro-Hyp treatment. Additionally, the expression of RunX1 and osteocalcin decreased with Pro-Hyp treatment. No RT-PCR analysis was performed on CH-treated cells. In summary, this study was one of the first to clearly establish that Pro-Hyp is a chief bioactive component associated with the observed clinical efficacy of CHs in the treatment of OA, specifically on cartilage tissue
[27][63].
Another BAP derived from CHs is Gly-Pro-Hyp, which has been suggested to be involved in platelet aggregation, which was recognized by platelet glycoprotein VI
[32][33][67,68]. This interaction is unique; Gly-Pro-Hyp occurs rarely in other proteins, except for collagen, and glycoprotein VI is thought to be expressed solely by platelets. Furthermore, this tripeptide, which was generated by hydrolyzing porcine, bovine, fish, and chicken collagen with
Streptomyces collagenase has been shown to inhibit the activity of DPP-IV, which has been associated with diabetes
[33][68]. The production of Gly-Pro-Hyp depended on the source of the collagen. Porcine and bovine collagen yielded greater Gly-Pro-Hyp content compared to fish and chicken collagen after hydrolysis. Other generated peptides (Gly-Ala-Hyp and Gly-Pro-Ala) were assessed for their DDP-IV activity, although only Gly-Pro-Hyp showed bioactivity. This peptide might prove to be an important health modulator in OA as patients diagnosed with diabetes are at an increased risk of developing arthritis
[34][35][36][10,69,70].
Preliminary in vitro studies using bone marrow macrophages differentiated into OCs showed that collagen decreased the number of differentiated OCs, i.e., positively tartrate-resistant acid phosphatase (TRAP) stained cells. Other reports have shown that CHs from animal skins decreased OC resorption area but did not affect OC growth
[37][71]. A more recent study by N’deh et al. (2020) used OC precursor RAW 264.7 cells and demonstrated that collagen extract from chicken decreased the mRNA levels of TRAP and cathepsin k
[38][72]. Collagen from Yeonsan Ogye chicken flesh was extracted in a pressure chamber at 121 °C, 0.5 mPa for 30 min, filtered, and then lyophilized until used for the study.
The effects of CHs on OBs have been more thoroughly investigated, although the mechanisms of action remain to be fully established. Previous work using bovine collagen on a pre-osteoblast cell line (MC3T3-E1 cells) observed changes in gene expression, primarily an increase in Runx2
[39][22]. The increased expression of ALP activity and mineralization was also observed. Another in vitro study using MC3T3-E1 cells helped establish that the BAP and Pro-Hyp promoted osteoblastic differentiation, but not proliferation
[40][73]. Pro-Hyp treatment was shown to upregulate both osteoblastic differentiation genes Runx2 and Osterix, as well as Col1a1. The application of the collagen tri-peptide Gly-Pro-Hyp on MC3T3-E1 cells also showed upregulated protein expression of Runx2, Osterix, ALP, and Col1a1 in a dose-dependent manner
[41][23]. Using the human osteoclastic MG-63 cells, CHs were shown to stimulate ALP activity, calcium deposition, and collagen synthesis
[42][74]. The CH was porcine-sourced and hydrolyzed with a combination of protamex + flavorzyme at 50 °C for 12 h, fractioned and filtered (<3 kDa), and freeze-dried.
5. Digestion and Bioavailability of CHs and CH-Derived Peptides
When consumed, CHs, BAPs, and any other nutraceuticals or medications taken orally must undergo digestion and absorption before exerting their bioactive effects [43][44][17,75]. It is mainly in the small intestine (SI) that proteins are broken down into peptide and AA components. However, the final breakdown of proteins and larger peptides to their smaller components occurs on the surface of resident intestinal enterocytes by brush border enzymes. The BAPs released after protein and peptide digestion are absorbed by villus enterocytes [45][76]. The remaining food components and nutrients that are not absorbed in the SI travel to the large intestine. In these colonic regions, non-dietary carbohydrates, proteins, peptides, and AAs can be fermented by resident microbiota [46][47][77,78].
After digestion, BAPs undergo first-pass metabolism, which mediates the entry of bioactive molecules into the systemic circulation
[48][49][79,80]. This process is defined by the absorption of metabolic compounds at the level of the intestinal epithelium, followed by hepatic metabolism, before being released into the blood. The additional release of BAPs after CH digestion may occur, as the CH peptide components can be broken down into smaller BAPs and AAs in the stomach and SI. Regardless of the extent of digestion, the bioactivity of CH-derived BAPs, and therefore clinical efficacy, depends heavily on their bioavailability, which is the proportion that reaches the systemic circulation unaltered upon oral ingestion
[44][48][75,79]. Peptide bioavailability remains one of the greatest factors affecting bio-potency, and the digestion and absorption of different CHs can differ greatly, which may lead to altered CH bioactivities
[8][50][44,81]. Large MW peptides are less effectively absorbed than lower MW peptides, so CHs with lower MWs are more likely to be absorbed to exert their bioactivity
[4][10][16,46]. There are four main routes of peptide absorption by intestinal enterocytes: (1) passive diffusion; (2) paracellular transport; (3) transcytosis; and (4) carrier-mediated transport (active transport)
[44][75].