Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Iwona Kowalczyk and Version 3 by Jessie Wu.
Gemini surfactants are a modern variety of surfactants with unique properties and a very wide range of potential applications. Hexamethylene-1,6-bis(N-dodecyl-N,N-dimethylammonium bromide) is one such representative compound that is a better alternative to a single analogue. It shows excellent surface, antimicrobial, and anticorrosion properties. With a highly efficient synthetic method and a good ecological profile, it is a potential candidate for numerous applications, including biomedical applications.
- gemini surfactants
- 12-6-12
- synthesis
- properties
- antimicrobial activity
- applications
1. Introduction
Interactions at the interface are of fundamental importance in chemistry, physics, and biology. The thermodynamic parameters of these interactions can be modulated using surfactants. Owing to their amphiphilic structure, which comprises a hydrophilic and hydrophobic part, surfactants decrease the surface tension or interfacial tension between two liquids, a liquid and gas, or a liquid and solid. Hence, they can act as wetting, dispersing, and emulsifying agents. These properties enable the production of a large number of products required in household chemistry and cosmetic, pharmaceutical, agrochemical, petrochemical, textile, and paper industries.
The dynamic development of surfactant chemistry is a continuation of what was initiated by nature, which created the biosurfactants necessary for the functioning of living organisms. Biosurfactants usually refer to surfactants of microbial origin and comprise lecithin, rhamnolipids, sophorolipids, and emulsan [1][2][1,2]. Biosurfactants, in addition to their intrinsic role, have a wide range of technical applications. For instance, they can solubilise hydrocarbon contaminants and can be used in enhanced oil recovery [3][4][3,4]. Owing to their low toxicity and biodegradability, biosurfactants are extremely valuable products from the viewpoint of environmental protection. The global market size for biosurfactants reached a value of more than USD 2.33 billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 5.8% between 2023 and 2028, reaching a projected value of USD 3.27 billion by 2027 [5][6][5,6]. Unfortunately, the number of available biosurfactants as well as the range of their applications does not meet the requirements expected from surfactants.
Currently, the widest groups of surfactants that meet the application requirements are nonionic, anionic, cationic, and amphoteric synthetic surfactants. The global demand for these surfactants, including soaps, exceeds 20 million tons per year [6]. The global surfactant market stood at a value of approximately USD 41.84 billion in 2022. The market is further expected to grow in the forecast period 2023–2030 at a CAGR of 4.6% to reach a value of USD 59.95 billion by 2030 [6]. However, the large volume of surfactants disposed into the environment, despite the wastewater treatment processes, is a serious burden and threat to the environment. Thus, owing to the increasing demand for surfactants, the development of new and more effective surfactants is extremely important.
Cationic gemini surfactants have emerged as a result of the research conducted in this field over the last several years. Gemini surfactants are compounds that are composed of two hydrophilic head groups and two hydrophobic tails linked by a spacer at the head groups or close to them (Figure 1). The spacers can be flexible (methylenes) or rigid (aromatic structures). The type of spacer (short or long, hydrophilic or hydrophobic) influences the shape of the micelles. The neutral charge of the molecule is retained in the presence of organic or inorganic counterions [7][8][9][7,8,9].
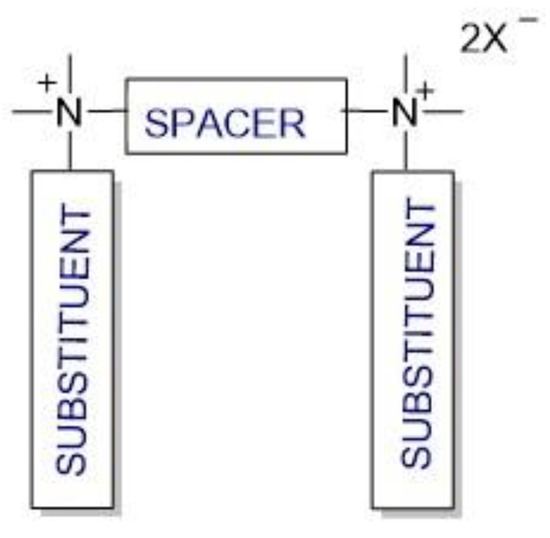
Figure 1.
Structure of gemini surfactants.
The critical micelle concentration (CMC), surface tension (γ), and minimal inhibitory concentration (MIC) of gemini surfactants are a dozen times lower than those of monomeric surfactants. The unique properties of gemini surfactants with a wide range of hydrophilic–lipophilic balance (HLB) render them a very useful and innovative material in chemistry (e.g., for corrosion inhibition, micellar catalysis, nanoparticle synthesis, preparation of supramolecular solvents, nanoemulsion preparation, and synthesis of precisely defined polymers) [10][11][12][13][14][10,11,12,13,14], medicine (as biocides, drug carriers, and capping agents for metal nanoparticles with biocidal properties or for preparing nonviral gene delivery systems and inducing protein conformational changes) [15][16][17][18][19][20][15,16,17,18,19,20], and optoelectronics (through a spatial network of well-dispersed molecules) [7]. Gemini surfactants are a modern solution for all areas that need surfactants, including households, detergents, personal care, institutional and industrial cleaning, food processing, plastics, paints and coatings, oilfield chemicals, petrochemistry, agricultural chemicals, adhesives, and textiles [20].
2. Structure and Synthesis
12-6-12 consists of two N-dodecyl-N,N-dimethylammonium units connected with a chain of six methylene groups as a spacer. Bromine ions are present as counterions (Figure 23). This compound has been classified under chemical abstracts service (CAS) number 18507-15-8 and is a dimeric analogue of N-dodecyl-N,N,N-trimethylammonium bromide (DTAB), which is commonly used as a microbiocide.
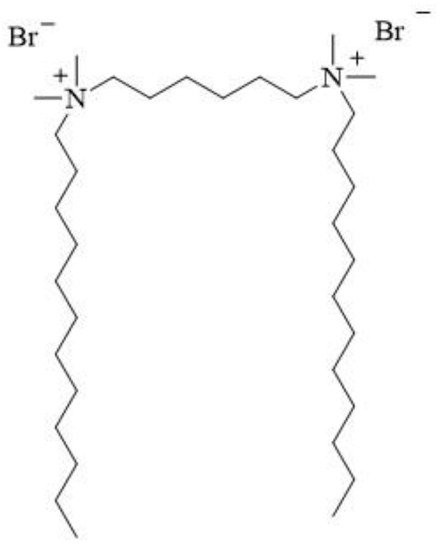
Figure 23.
Chemical structure of 12-6-12.
12-6-12 is one of the first compound to be classified as a double quaternary ammonium salt and defined as a gemini surfactant. This compound is synthesised via the quaternisation of amines, a process referred to as the Menschutkin reaction:
-
alkylation of hexamethylene-bis(N,N-dimethylamine) with 1-dodecylbromide (Figure 34a);
-
linking of N-dodecyl-N,N-dimethylamine with 1,6-dibromohexane (Figure 34b).
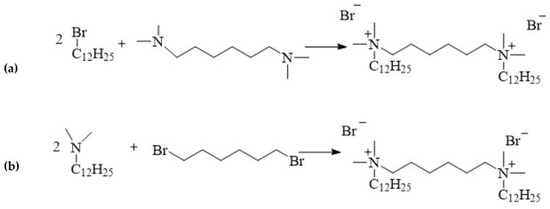
Figure 34.
Synthesis of 12-6-12.
The synthesis of 12-6-12 was first reported in 1968 by Sindenko et al. [21]. Stoichiometric amounts of dodecylbromide and hexamethylene-bis(N,N-dimethylamine) were reacted in ethanol for the synthesis (yield 70%) [21]. Regardless of the synthetic pathway, the quaternisation reaction always follows the SN2 nucleophilic substitution mechanism. The rate of the reaction depends on the concentrations of both reagents, although there are reports of the use of excess amine [22][23][24][25][22,23,24,25] or bromide [26][27][28][26,27,28]. Typically, polar solvents such as alcohols (methanol [27][29][27,29], ethanol [30][31][30,31], isopropanol [32]), acetone [19][23][33][19,23,33], or acetonitrile [22][24][34][22,24,34] are used in the synthesis of 12-6-12. These reactions occur at the boiling point of the solvent. The type of solvent used determines the reaction time because SN2 reactions are the fastest in polar aprotic solvents such as acetonitrile. Replacing ethanol with acetonitrile reduces the reaction time from 24 to 5 h [25][35][25,35]. 12-6-12 can also be synthesised in solvent mixtures such as the acetonitrile/toluene mixture [36]. The reaction time can be shortened using microwave radiation [37]. However, the most economical and ecological approach is to synthesise 12-6-12 under stoichiometric conditions at room temperature without a solvent [38]. In this case, good yields of over 90% can be achieved using small amounts of reagents over a reaction time of 0.5 h [38]. Solvent-free synthesis can also be easily carried out on a large scale.
To obtain pure 12-6-12, the crude product can be crystallised from acetonitrile [35] or from dichloromethane–diethyl ether [39], acetone–methanol [40][41][40,41], ethanol–ethyl acetate [23], ethanol–diethyl ether [24], acetone–ethanol [21], and acetone–ethyl acetate [25] mixtures.
36. Applications
Surfactants are ubiquitous, being key components in a diverse range of complex industrial processes and utilitarian products such as dispersants, solubilisers, emulsifiers, demulsifiers, foaming agents, wetting agents, disinfectants, corrosion inhibitors, antistatic agents, and viscosity modifiers. In the last few decades, significant efforts have been made for the synthesis of new gemini surfactants, fuelled by their remarkably improved physicochemical properties that can be achieved by the modification of structural factors [42][92]. Cationic gemini surfactants have wide applications due to their excellent surface activity [7][20][7,20]:
-
in foaming, agrichemical spreading aids, and cleaning;
-
from industrial to personal care applications;
-
phase transfer catalyst;
-
bleach activator;
-
as hair conditioners and fabric softeners.
12-6-12 was first described 55 years ago, but the increased application interest of this compound can be defined in the 21st century. 12-6-12 can be used in several practical applications (Figure 48).
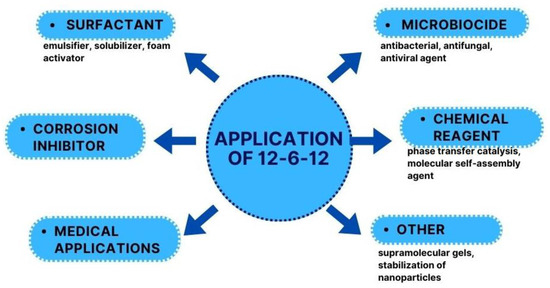
Figure 48.
Applications of 12-6-12.
12-6-12 is characterised by excellent surface, antimicrobial, and anticorrosion properties. As a compound that can be obtained economically and ecologically, it is an ideal product that has many industrial applications, including bioapplications (Table 19).
Table 19. Potential bioapplications of 12-6-12.
| Application | References |
|---|---|
| Enhanced drug delivery | [43][44] |
| Drug carrier | [45] |
| Stabilization of drugs | [46] |
| Detection of DNA | [33] |
| Nonviral gene delivery agent | [47][48][49][50] |
| Eradication of biofilm | [51][52] |
| Filtering nonwovens | [53] |
| Bioactive cellulose products | [54][55] |
| Addition to DNA filtration | [56] |
Potential bioapplications of 12-6-12.
Moreover, 12-6-12 can be used in chemical synthesis for the preparation of supramolecular gels [57][101] and as a nanoparticle stabiliser [28][41][58][28,41,102], an interfacial transfer catalyst [30][31][59][60][61][30,31,103,104,105], and a molecular self-assembly agent [62][106].
In conclusion, quaternary ammonium gemini surfactants are known for their multifunctional utility properties such as antimicrobial, surfactant, and anticorrosion properties. 12-6-12 has all the above mentioned properties of gemini surfactants and can be obtained in a one-step reaction, which is an indisputable and economically justified advantage over other gemini surfactants. Compared to its monochain counterpart, it shows better surface, antimicrobial, and anticorrosion activity and is characterised by a lower toxicity. The use of this compound in concentrations much lower than DTAB while providing the same utility effect makes 12-6-12 a candidate in many applications. Initially, this compound was used in applications as typical quaternary ammonium salts as an interfacial transfer catalyst. Currently, this compound finds potential applications in many different areas of life. Particularly noteworthy is the testing of this compound in biomedical applications. Due to its anticorrosive, biocidal, dispersing, and detergent properties, 12-6-12 can be used as an additive to car fuels and as an anticorrosion and antimicrobial agent in oil pipelines, smart anticorrosion coatings, etc. Hence, the possibility of producing it in large quantities in a one-step, waste-free synthesis is a huge advantage over other gemini surfactants.
