Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vishnu D. Rajput and Version 2 by Jessie Wu.
Unlike other secondary metabolites, phenolic compounds are found in almost all plant cells. They hold functional significance not at the cell level, but at the level of the whole plant. The central enzyme of phenylpropanoid metabolism (phenylalanine ammonia-lyase) is inducible: due to the induction of the expression of coding genes, its activity increases sharply under the influence of stress factors.
- heavy metals
- anthropogenically transformed environment
- phenolic compounds
- flavonoids
- anthocyanins
1. Flavonoids
Flavonoids play a significant role in protecting plants against various kinds of stresses of a biogenic and abiogenic nature [1][2][3][3,12,15]. Appropriate literature analysis shows that the impact of technogenic pollution can affect the accumulation of these substances in plants in different ways.
A number of authors have put forward the assumption that flavonoids contribute to the formation of plant tolerance to adverse environmental conditions. An increase in the content of these metabolites is one of the nonspecific reactions to stress [2][4][5][6][11,12,16,17]. Several pharmacopeia authorities fixed the HM concentration in different herbal formulation are shown in Figure 1.
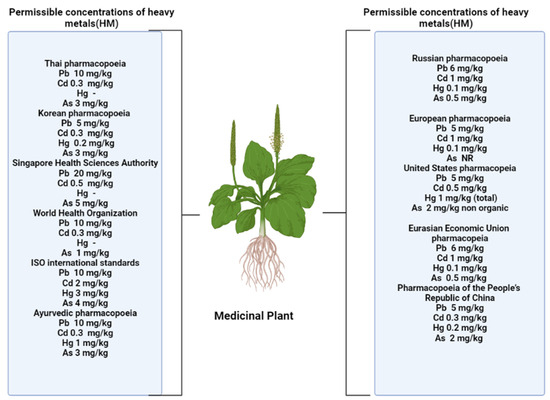
Figure 1. Permissible levels of heavy metals in accordance with standards established in various nations [7].
Literature describes the possibility of using HM to activate the phenylpropanoid pathway. Induction of the synthesis of flavonoids was noted in the tissues of Lemna gibba L. plants under the influence of Cu2+, in the calluses of Linum isitatissimum L. and Camellia sinensis (L.) Kuntse treated with cadmium preparations, in Phaseolus coccineus L. treated with Cd2+ and Cu2+ [5][8][16,18]. In the study by Santiago et al. copper sulfate was used to induce the synthesis of phenolic compounds in Phyllanthus tenellus Roxb. [1][3]. The example of Spinacia oleracea L., showed that mercury salts treatment leads to an increase in the content of flavonoids in leaves due to a decrease in the total amount of phenolic compounds, which is explained by the effect of this toxicant on the phenylpropanoid pathway [9][19]. In the needles of Larix sibirica Ledeb, an increase in the total content of phenolic compounds (by 50–55%), flavonoids (by 1.5–1.8 times), catechins (by 1.9–2.5 times), and proanthocyanidins (by 45%) was noted compared with the background level. The described effects were observed at weak, moderate, and strong pollution levels due to large aluminum plant emissions, while at a critical level of pollution, the content of these metabolites decreased [10][20]. In the study of plants growing along the highways of the large city of Donbass, an increase in the concentration of flavonoids was revealed in the flowers of Sambucus nigra L., the fruit of Rosa lupulina Dubovik, as well as in the fruit, leaves, and flowers of Crataegus fallacina Klokov due to a decrease in the content of other phenolic compounds in the fruits of this species Crataegus [11][21].
There are often studies that describe directly opposite phenomena. The revealed decrease in the level of flavonoids under the influence of technogenic load in Tussilago farfara L., Trifolium rubens L., and Vicia cracca L. is explained by the participation of these metabolites in HM chelation, their antioxidant function, oxidation to quinones under the action of free radicals, and suppression of biosynthesis by toxicants due to damage to the structure of enzymes (synthetase and reductase) by reactive oxygen species (ROS) or HM [12][13]. There is a probable relationship between the lower photosynthetic capacity of Tibouchina pulchra Cogn. in the conditions of environmental pollution from emissions of fertilizer producers, metallurgical plants and the chemical industry, changes in carbohydrate metabolism, and a decrease in the concentrations of phenolic compounds and tannins [13][22]. At the same time, when exposed to industrial air pollution in a large city in Latin America (emissions from the chemical and steel industries, ferrous metallurgy, the production of fertilizers and ceramics) with high concentrations of fluorine, particulate matter, and sulfur dioxide, no significant differences were found in the total amount of flavonoids in the leaves of Psidium guajava L. compared with plants growing in an uncontaminated area [14].
In the leaves and flowers of Tanacetum vulgare L., Chamaenerion angustifolium (L.) Holub., leaves of Linaria autiloba Fisch. ex. Reichenb., Artemisia mongolica (Bess.) Fisch. ex. Nakai, Artemisia jacutica Drob., Inula britannica L., Taraxacum ceratophorum (Ledeb.) DC., Achillea millefolium L., a significant decrease in the concentration of flavonoids was revealed in conditions of cement dust pollution (in the vicinity of a cement plant) [3][15]. In the study of plants growing along the highways of the capital of Donbass, a decrease in the concentration of flavonoids was found in the leaves of Cotinus coggygria Scop, the fruit of Rosa corymbifera Borkh., Sorbus aucuparia L. and Sorbus intermedia (Ehrh.) Pers [15][23].
The study of the characteristics of flavonoid metabolism in wild plants forced to adapt to an environment polluted by industrial emissions is also important for studying the formation of tolerance in specific species to technogenic stress and can be used to diagnose the state of the environment. In the study of plants Trifolium pratense L., Artemisia absinthium L., Taraxacum officinale L., and Achillea millefolium L. under the conditions of their long-term adaptation to technogenic pollution (at the Ufa oil refinery and its surroundings), a lower content of flavonoids in the aerial part was found compared with plants from an ecologically clean zone. The results obtained correlated with the level of soil contamination. At the same time, another pattern was revealed in the roots of the studied plants–the content of flavonoids was higher for plants from the contaminated area [16][24]. It is shown that under conditions of varying degrees of pollution in the plant, both an increase and a decrease in the content of flavonoids can be observed (for example, Matricaria chamomilla and Poa pratensis), as the authors suggest, this indicates a wide range of resilience of these species [12][13].
It is important to note that the method of quantification of flavonoids (different authors often use different methods) can affect the results obtained. It should also be taken into account that under conditions of technogenic load, hydrolysis of glycosidic forms of flavonoids can occur, and can lead to an increase in their recorded content [12][13]. The place of harvesting of the control plants with which the comparison is made is also important. As such, various researchers use a botanical garden, a park, the countryside, as well as pharmacy plant materials. Often, an article does not provide a detailed description of the environmental conditions for the growth of control plants, although the content of biologically active substances is affected not only by the technogenic load, but also by the composition of the soil, humidity, temperature, amount of solar radiation, etc. This makes it difficult to interpret the results.
Mechanism of Heavy Metals in Enhancement of Phenolic Compound
A significant worldwide concern is the release of HM into the ecosystem as a result of various anthropogenic activities. The growth of medicinal plants in soil enriched with HM can ultimately impact the biosynthesis of phytocompounds [17][18][25,26]. These HM is be responsible for a higher level of ROS generation. Due to increasing ROS generation, it may cause oxidative stress, deterioration of biomolecules, and breakdown of the lipid membrane.
On the other hand, ROS also function as messenger molecules, which may trigger other downstream signaling channels that regulate phytocompound synthesis inside medicinal plants. It has been discovered that higher levels of ROS brought on by HM stress cause mitogen-activated protein kinases (MAPKs) to become active. Directly phosphorylating the higher expression of transcription factors like CrMAPK3 and ORAC3, in turn causes an increase in quantity of various phyto-compounds of Catharanthus roseus under Ag+ treatment via increasing expression of GS, STR, and CrPRX as well as DAT genes [19][27]. It is also widely known that HM stimulates the expression of other signaling molecules such as ethylene, which participates in the regulation of pathways that lead to the synthesis of tropane alkaloids (hyoscyamine and scopolamine) in Brugmansia candida hairy root cultures subjected to Ag+ [20][28]. A diagrammatic representation of the signaling route involved in the buildup of phytochemicals brought on by heavy metals is represented in Figure 2.
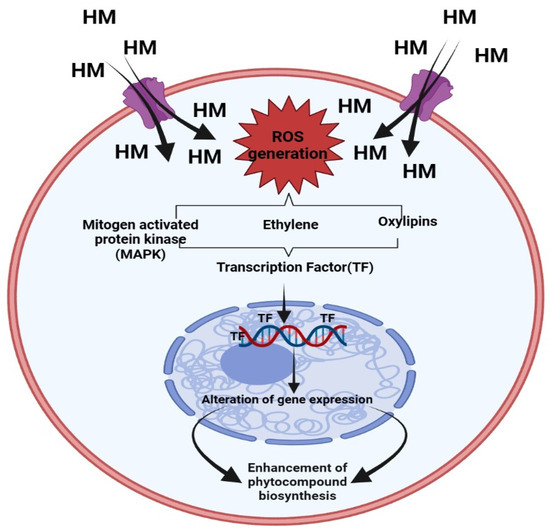
HM stress leads to the production of toxic ROS in plants, which in turn results in poisoning as well as diminished plant development [21][22][23][29,30,31]. Several studies reveal that HM is responsible for the biosynthesis of polyphenols, which act as shields for plants against oxidative damage [24][25][26][32,33,34]. The finding reveals that the amount of polyphenols in plants has been shown to be significantly boosted by metal excess [26][27][34,35], which aligns well with the finding that polyphenols may accelerate the metallic chelation mechanism and assist in reducing the amount of damaging free radicals in plant cells [28][29][36,37]. High exposure to HM causes the accumulation of bioactive compounds like flavonoids that help the defense systems of plants [30][31][32][38,39,40]. Under metal stress the level of transcription of the genes of such enzymes as phenylalanine ammonia lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), cinnamate 4-hydroxylase (C4H), 4-coumarate: CoA ligase (4CL), flavanone3-hydroxylase (F3H), flavonoid 30-hydroxylase (F30H), flavonoid 305 0-hydroxylase (F305 0H), flavonol synthase (FLS), flavone synthase (FNS), UDP flavonoid glycosyl-transferase (UFGT), isoflavone synthase (IFS), isoflavone reductase (IFR), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS) [32][40].
In the phenolic/ascorbate-peroxidase cycle, flavonoids are known for their ability to scavenge H2O2 and are thought to be essential [33][34][35][41,42,43]. Several significant enzymes, including shikimate dehydrogenase (SKDH) and glucose-6-phosphate dehydrogenase (G6PDH), facilitate the biochemical process needed to produce key substrates of phenylpropanoid pathways [36][44]. By increasing the activity of important biosynthesis enzymes such as PAL, SKDH, G6PDH, and CADH, heavy metals drive the phenylpropanoid biosynthetic pathway in plants [36][37][44,45]. The effects of metal stress on plants’ phenolic content are summarized in Figure 3. Several major flavonoids like catechin, kaempferol, apigenin and vitexin are shown in Figure 4.
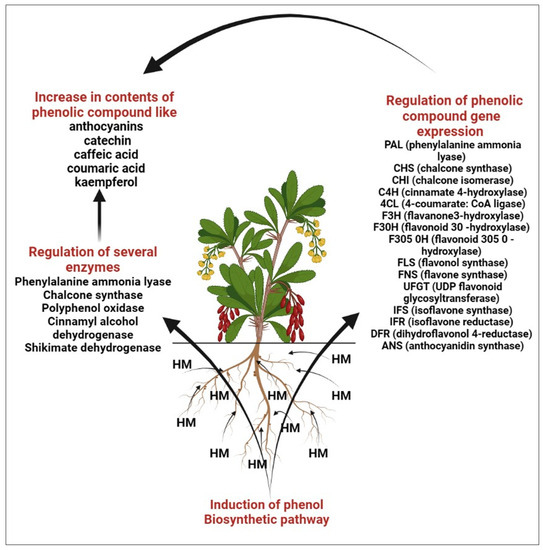
Figure 3.
Role of heavy metal in enhancement of phenolic compound.
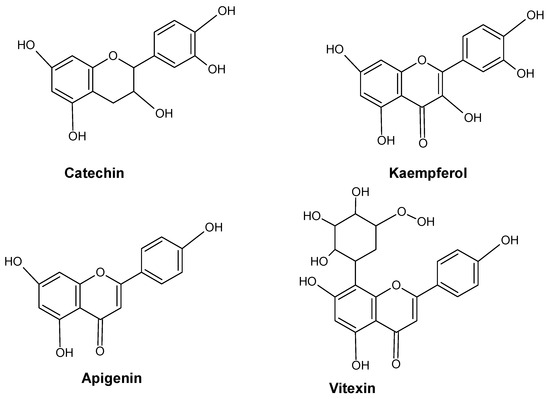
Figure 4.
Major flavonoids in plants.
2. Anthocyanins
Anthocyanins deserve special attention as a group of flavonoids due to their high physiological activity. It is suggested that, in contrast to pigmentation in flowers and fruit, the accumulation of these metabolites in leaves is a response to oxidative stress [38][46]. In addition to the direct neutralization of free radicals (their antiradical activity exceeds that of other classes of flavonoids, ascorbate, and α-tocopherol), anthocyanins are capable of chelating metal ions [39][47]. Anthocyanins located in vacuoles are able to neutralize peroxides, which is especially important due to the fact that the absence of ascorbate peroxidase in cell vacuoles does not allow ascorbic acid to participate in the detoxification of ROS [40][48]. Not vacuolar, but cytosolic anthocyanins likely make a more significant contribution to the antioxidant system; they are located closer to the sources of superoxide radical synthesis. The degree of contribution of anthocyanins to the antioxidant system of the plant differs among other low molecular weight antioxidants in different species. In some plants, they are the main antioxidants, while in others, a high level of anthocyanin biosynthesis is not necessary for protection against oxidative stress [41][49].
The biosynthesis of anthocyanins is a nonspecific response to stressful growing conditions (low temperatures, drought, HM pollution, oil pollution, and ionizing radiation), and can be explained by the expression of genes associated with their synthesis [42][43][44][5,50,51]. The content of anthocyanins is proposed to be used to assess the physiological state of plants [42][5].
An analysis of literary sources shows that most researchers note the accumulation of anthocyanins in the leaves of plants growing under conditions of technogenic pollution. Under conditions of oil pollution, an increase in the content of anthocyanins was detected in the leaves of Leymus arenarius L. Hochst, Ammophila arenaria L. Link, Lathyrus maritimus Bigel., Petasites spurious (Retz) Reishb., Salix daphnoides Will., Salix caprea L., and Salix aurita L. [42][45][5,52]. Under conditions of pollution with railway pollutants (petroleum and transmission oils), an increase in the concentration of anthocyanins was found in the leaves of Geum urbanum L., Anthriscus sylvestris L., Glechoma hederacea L., Taraxacum officinalis L., Dactylis glomerata L., and Achillea millefolium L. (by an average of 5.2 times). Cultivation of Cassia grandis L. plants on a mining waste substrate for 4 months led to an increase in the concentration of anthocyanins in leaves and roots (due to a decrease in the content of chlorophyll a, b and carotenoids), where the control plants were grown on a commercial substrate [44][51]. In urban conditions, an increase in the content of anthocyanins in the leaves of Psidium guajava ‘Paluma’ was found [46][53].
In the conditions of the urbanized environment of Donbass, anthocyanin accumulation was observed in the fruitof Rosa corymbifera, Sorbus aucuparia, and Sorbus intermedia, flowers and leaves of Crataegus fallacina, while in the fruit of Rosa lupulina and flowers of Sambucus nigra their concentration decreased [11][15][21,23]. With the intensification of urban air pollution by automobile pollutants, the content of anthocyanins increased in the needles of Picea abies (L.) Karst., leaves of Tilia cordata Mill., Taraxacum officinale Webb., Plantago major L., Sorbus aucuparia L., and Acer platanoides L. An increase in the concentration of cadmium in soil stimulated the accumulation of anthocyanins in the leaves of Lathyrus maritimus Bigel., Secale cereale L., Lolium perenne L., Festuca rubra L., and Poa pratensis L. was due to a decrease in the total content of phenolic water-soluble antioxidants [42][45][5,52].
An increase in the anthocyanin content of a plant’s leaves is frequently a sign of environmental stress. For example, Vasconcelos et al. showed the accumulation of anthocyanins in the leaves of Zea mays L. plants growing under conditions of phosphorus deficiency [43][50]. Some anthocyanins are shown in Figure 5.
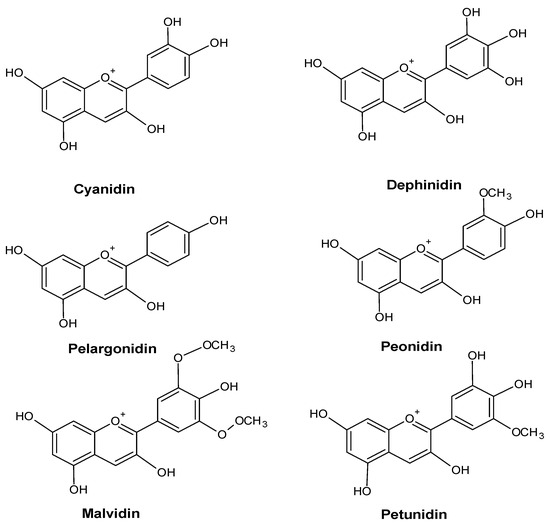
Figure 5.
Major anthocyanin in plants.
3. Tannins
Many studies have noted a decrease in the content of tannins with an increase in the intensity of technogenic pollution, which is explained by the suppression of the processes of synthesis and the ability of metals to reduce the content of tannins by precipitation. In particular, a decrease in the level of tannins was found in the leaves of Acer negundo L., Acer platanoides L., and Tilia cordata, growing on plantations in sanitary protection zones of industrial enterprises and along the highways, in the leaves of Prunus padus L., collected next to a metallurgical enterprise and by a combined heat and power plant, in the leaves of Plantago major L., growing by a highway, in the leaves of Betula pubescens spp., growing near a steel plant, and in the aerial part of Achillea nobilis L. and Melampyrum pratense L. in the area of industrial installations of a gas processing plant [46][47][48][53,54,55]. The chelating ability of tannins is proposed to be used to remove metal ions from wastewater [49][56].
Under urban conditions, an increase in the content of tannins was found in the leaves of Psidium guajava ‘Paluma’ [46][53]. In the leaves of Acer platanoides L. plants growing on the plantations in the sanitary protection zones of the casting and blacksmith plants and along the highways, an increase in the activity of polyphenol oxidase was revealed compared with the control (plantations of the forestry), while the content of condensed tannins decreases, which indicates the active participation of tannins in adaptive reactions of plants associated with the mechanisms of neutralization of pollutant effects [47][54].
Katoh et al. showed that the inhibition of the synthesis of condensed tannins in the leaves of Cryptomeria japonica D. Don., caused by air pollution in the vicinity of a steam power plant, is due to disturbances in the formation of aromatic rings from 3-dehydroshikimic acid, i.e., the conversion of shikimic acid to phenylalanine and tyrosine [50][57]. The content of tannins in the leaves is negatively correlated with the levels of soluble sulfate. Under the influence of toxicants, a decrease in the photosynthetic activity of C. japonica is observed. The authors suggest that in this case the main part of free glucose is used for growth and primary metabolism, which can explain the decrease in the intensity of secondary metabolism, including synthesis of tannins [51][58].
Along with the above results, there are also other studies showing an increase in the biosynthesis of tannins in contaminated areas, which is explained by the protective reaction of plants to adverse environmental conditions. This is observed in the leaves of Fragaria viridis L. plants growing on the territory of the gas processing plant, and in Plantago samples taken near the highway [46][48][51][53,55,58]. Under the conditions of the urbanized environment of Donbass, a decrease in the concentration of tannins was found in the medicinal raw material of most of the studied species (Rosa lupulina, Sorbus aucuparia, Sorbus intermedia, Sambucus nigra, Tilia cordata, Cotinus coggygria), while in the fruit of Rosa corymbifera, leaves and flowers of Crataegus fallacina its content increased [11][15][21,23].
Thus, the dynamics of the content of tannins under conditions of pollution requires careful study for each individual type of medicinal plants in a particular region. The revealed discrepancy in the results observed by different authors can also be explained by the predominant group of tannins (hydrolysable or condensed) in the studied plant material and, accordingly, by different ways of their biosynthesis and properties, which determine the reaction of their metabolism to technogenic impact. A good example of this is the work that analyzed the concentration of tannins in the fruit of Rosa L., grass Tussilago farfara L. and Plantago major L., harvested near the highway [48][55]. A decrease in the concentration of hydrolysable tannins compared with the control (forest) was revealed, which is explained by their binding to HM, and an increase in the content of condensed tannins due to their protective function (Figure 6).
Figure 6.
Major tannins in plants.
4. Phenolcarboxylic Acids
Free phenol carboxylic acids, along with flavonoid aglycones, are the most physiologically active forms of phenolic compounds [52][4]. Of these, hydroxycinnamic acids are especially significant in pharmacy, having a variety of pharmacological effects on the human body. In plants, they are localized in the cell wall and play an essential role in the regulation of its physico-chemical properties. In the case of mechanical damage or penetration of pathogens, hydroxycinnamic acids, and lignin, by binding to non-phenolic polymers of cell walls, they contribute to their strengthening, prevent the penetration of pathogens and uncontrolled loss of water. The ability of these metabolites to bind HM ions into stable complexes was found. Interestingly, the use of exogenous hydroxycinnamic acids to increase plant tolerance when growing under conditions of HM intoxication is a promising direction in soil reclamation. High antioxidant activity was noted for chlorogenic and ferulic acids [5][52][53][4,16,59].
