Neuroendocrine neoplasms (NENs), also called neuroendocrine tumors (NETs), are relatively uncommon, heterogenous tumors primarily originating in the gastrointestinal tract. With the improvement in technology and increasing use of cross-sectional imaging and endoscopy, they are being discovered with increasing frequency. Advanced gastrointestinal endoscopic technique scan play a crucial role in the diagnosis and management of this rare condition.
Neuroendocrine neoplasms (NENs), also called neuroendocrine tumors (NETs), are relatively uncommon, heterogenous tumors primarily originating in the gastrointestinal tract. With the improvement in technology and increasing use of cross-sectional imaging and endoscopy, they are being discovered with increasing frequency. Advanced gastrointestinal endoscopic technique scan play a crucial role in the diagnosis and management of this rare condition.
- Neuroenocrine neoplasms
- NET
- Endoscopy
- Neuroendocrine tumors
- Gastrointestinal Endoscopy
1. Introduction
2. Role of Endoscopy in the Diagnosis, Staging, and Management of Nets Based on Location
2.1. Esophagus
Esophageal NENs (e-NENs) are rare, accounting for 0.2% to 1.3% of all GI NENs [6][7]. Lee et al. identified only 26 esophageal NENs among 2037 GI NETs [7]. Studies report a mean patient age of 60 years, with male preponderance [7][8]. Dysphagia, weight loss, and abdominal discomfort are common presenting symptoms [7][9]. NENs are sometimes discovered incidentally on esophagogastroduodenoscopy (EGD). They can range from low-grade carcinoid tumors, which have a good prognosis following resection, to high-grade NENs and large-cell or small-cell esophageal carcinomas that present as fungating masses [9]. Carcinoid syndrome is rarely seen on presentation since most tumors have a low degree of differentiation [9]. During EGD, low-grade e-NENs are generally seen as a single lesion in the lower third esophagus [7]. Esophageal neuroendocrine cancers (NECs) may be seen as exophytic polypoid masses, with or without ulceration and surface necrosis [10]. On white light imaging, surface redness may be seen due to vascularity. Narrow band imaging (NBI) may show abundant reticular vessels in carcinoid tumors. There is no specific TNM staging system for esophageal NECs owing to their rarity compared to esophageal squamous cell carcinoma and adenocarcinoma. They may be staged as ‘limited disease’ (LD) when confined to the esophagus or ‘extensive disease’ (ED) when they have spread beyond locoregional boundaries per the Veteran’s Administration Lung Study Group (VALSG) criteria [11]. Some authors used the 2009 American Joint Committee on Cancer (AJCC) TNM staging for esophageal squamous cell carcinoma for esophageal NECs [8]. Around 58% of esophageal NECs have evidence of regional lymph node involvement or widespread metastasis at diagnosis, with the liver, lung, and bone being the usual sites of distant spread [12]. EUS plays an essential role in identifying regional metastasis and estimating the extent of esophageal wall invasion for staging. Due to their rich vascularity, NENs are hyper-enhancing on contrast-enhanced EUS (CE-EUS). This hyper-enhancing nature differentiates them from hypo-enhancing leiomyomas, the most common esophageal smooth muscle tumor. Park et al., in their prospective study, reported that EUS-FNB was significantly faster and needed fewer samples than unroofing biopsy in diagnosing upper GI subepithelial tumors (SETs) (6 of 39 patients had esophageal SETs) [13]. A recent study concluded that EUS-FNA and key-hole biopsy (KHB) were equally effective in diagnosing upper GI SETs; however, the tissue sample was inadequate for determining the mitotic index [14]. Sanaei et al. found that EUS-FNB and single-incision with needle knife (SINK) were equally effective for upper GI SET sampling [15]. Mucosal incision-assisted biopsy (MIAB) is an upcoming technique, with a recent meta-analysis concluding that MIAB can be equally safe and effective as EUS-guided tissue acquisition [16]. A few case reports describe endoscopic management of esophageal NENs via polypectomy and endoscopic resection [17][18]. Definitive treatment guidelines for NETs have not yet been defined. Lee et al. proposed endoscopic resection (ER) for well-differentiated elevated tumors < 1 cm without regional lymph node metastasis or lymphovascular invasion [7]. Yagi et al. also similarly noted that ER could be considered for esophageal NENs < 10 mm in diameter, without ulceration or erosion, that are above the submucosal layer, given their low chance of lymph node metastasis [18]. Esophageal submucosal tumors (SMTs) can be safely resected by conventional endoscopic submucosal dissection (ESD) techniques, keeping in mind that the dissecting layer is thinner than in the case of epithelial lesions [19]. In their case series of 24 cases of foregut NEN lesions (1 in the esophagus, 24 in the stomach, and 4 in the duodenum), Li et al. reported histologically complete resection in 97% of cases using ESD [20]. The high R0 resection rate can help in accurate histological grading and staging and lower the chance of recurrence. In this series, there was no report of perforation, and only 1 case of delayed bleeding showing ESD can be a safe option for small e-NENs. Given the risk of perforation when ESD is performed for a submucosal tumor arising from the muscularis propria, Shim and Jung suggested that the depth of the lesion be evaluated first by EUS before treatment [21]. Endoscopic submucosal resection (ESD) may be preferred over endoscopic mucosal resection (EMR) as it allows for complete tumor resection with adequate horizontal and vertical margins. Due to the high en bloc resection rates, ESD also preserves the sample orientation for pathological assessment and has a lesser postoperative stricture risk [18]. Endoscopic enucleation with submucosal tunning (SMT) retains the mucosa and muscularis mucosa, reducing the chance of stricture formation [22]. Although endoscopic management can be the primary modality for the management of small esophageal NENs, larger tumors (>1 cm) warrant consideration for surgical resection and or chemotherapy. This is based on findings of increased rates of lymph node metastasis in NENs larger than 1 cm. However, given the rarity of esophageal NENs, these recommendations are based on the extrapolation of data from NENs in other locations of the GI tract. Esophageal NENs have high rates of being neuroendocrine carcinomas, and ESGE recommends treating them as esophageal adenocarcinomas [23]. Currently, there are no treatment guidelines that specifically address esophageal NENs, and treatment choice should be based on a comprehensive assessment of tumor size, grade, stage, the patient’s coexisting health conditions, and local expertise [24]. Future studies can be directed at analyzing the efficacy of EUS in identifying margins of NET before resection to ensure complete resection with clear boundaries. Patterns on CE-EUS for esophageal NEC can also be examined. Appropriate selection criteria for endoscopic resection are the need of the hour.2.2. Stomach
Gastric neuroendocrine neoplasms (g-NENs) are subdivided into three types based on etiology, pathogenesis, and prognosis. Type 1 and Type 2 gastric NENs result from neuroendocrine cell hyperplasia from pathologically elevated gastrin levels [2]. Type 1 g-NENs, which represent 70% to 80% of all g-NENs, occur in the setting of chronic atrophic gastritis (autoimmune gastritis and Helicobacter pylori-associated gastritis), resulting in chronic achlorhydria and resulting hypergastrinemia. Type 2 g-NEN is from hypergastrinemia due to pancreatic or duodenal gastrinoma (Zollinger–Ellison syndrome) and can be seen in patients with multiple endocrine neoplasia type-1 (MEN1) [2]. Persistent hypergastrinemia exerts a proliferative effect on the enterochromaffin-like (ECL) cells in the stomach. Type 1 and Type 2 gastric NENs usually follow an indolent course with a low risk of progression or metastasis. Most type 1 and type 2 g-NENs are histological grade, G1-G2, with a risk of metastatic disease of about 2–5% for type 1 and 10–30% for type 2, depending on various factors [25]. The risk for metastasis increases significantly when the size exceeds 1 cm [23]. Most Type 3 g-NENs, on the other hand, are sporadic, occur in the absence of hypergastrinemia; are histological grade G1, 2, or 3; NECs or mixed type; and are aggressive with local or hepatic metastasis present in up to 65% of patients on presentation. Gastric NENs are primarily diagnosed incidentally following endoscopy for non-specific symptoms such as dyspepsia, evaluation of anemia, or occult gastrointestinal bleeding. Type-1 g-NENs are typically small (<10 mm), often multiple, and are seen primarily in the gastric fundus or corpus [26]. Endoscopically, they appear as smooth, rounded, or polypoid submucosal lesions that can be yellowish or red in appearance (Figure 1) [23][26]. High-resolution magnifying endoscopy and narrow-band imaging (NBI) offer further characterization of these tumors. Most of the g-NEN surface is covered by normal mucosa, but often, a central depression can be seen where the gastric glands vanish. In this region, abnormally dilated subepithelial vessels with blackish-brown capillaries can be visualized under NBI imaging [26]. Type 2 g-NENs are similar to Type 1 g-NENs, but the adjacent gastric mucosa exhibits hypertrophic changes and often has coexisting areas of gastric or duodenal ulcerations. Type 3 gastric NENs usually occur as single, large (>2 cm) lesions that are often ulcerated.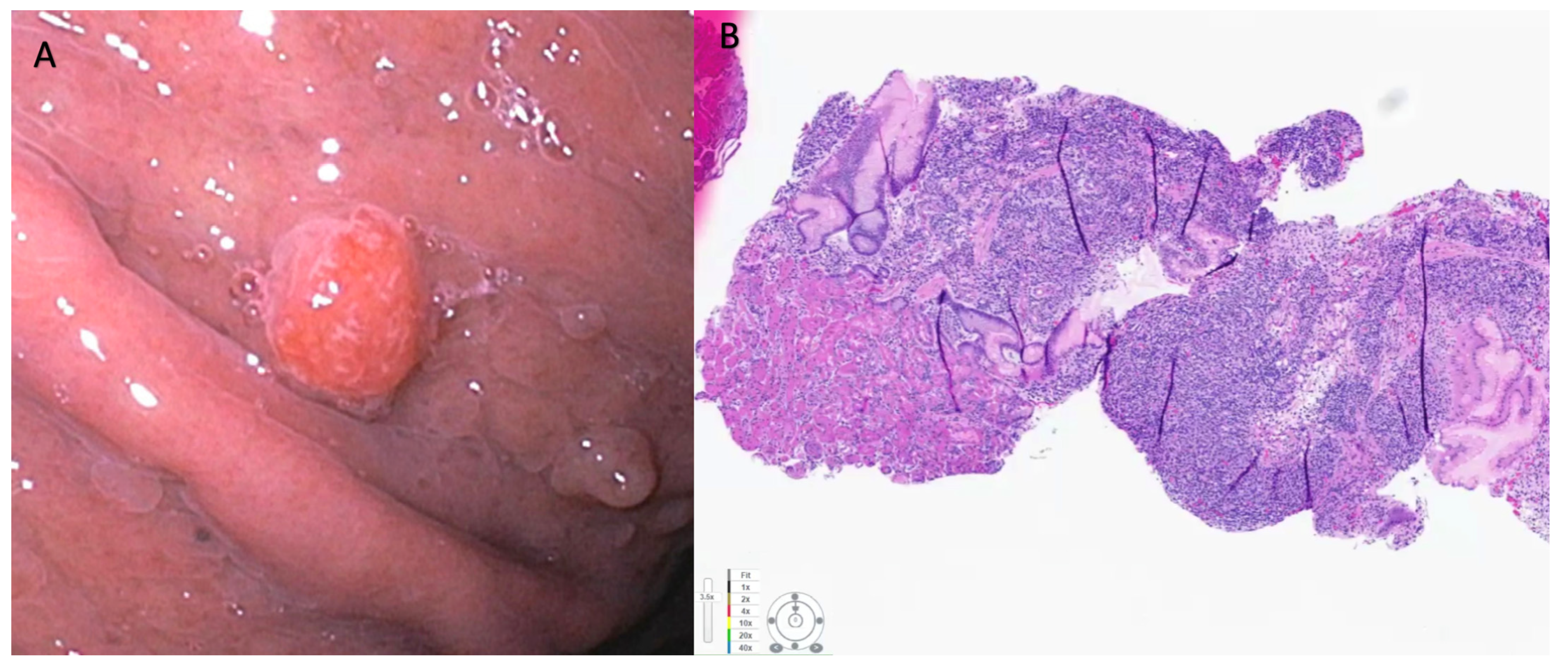
2.3. Small Intestine
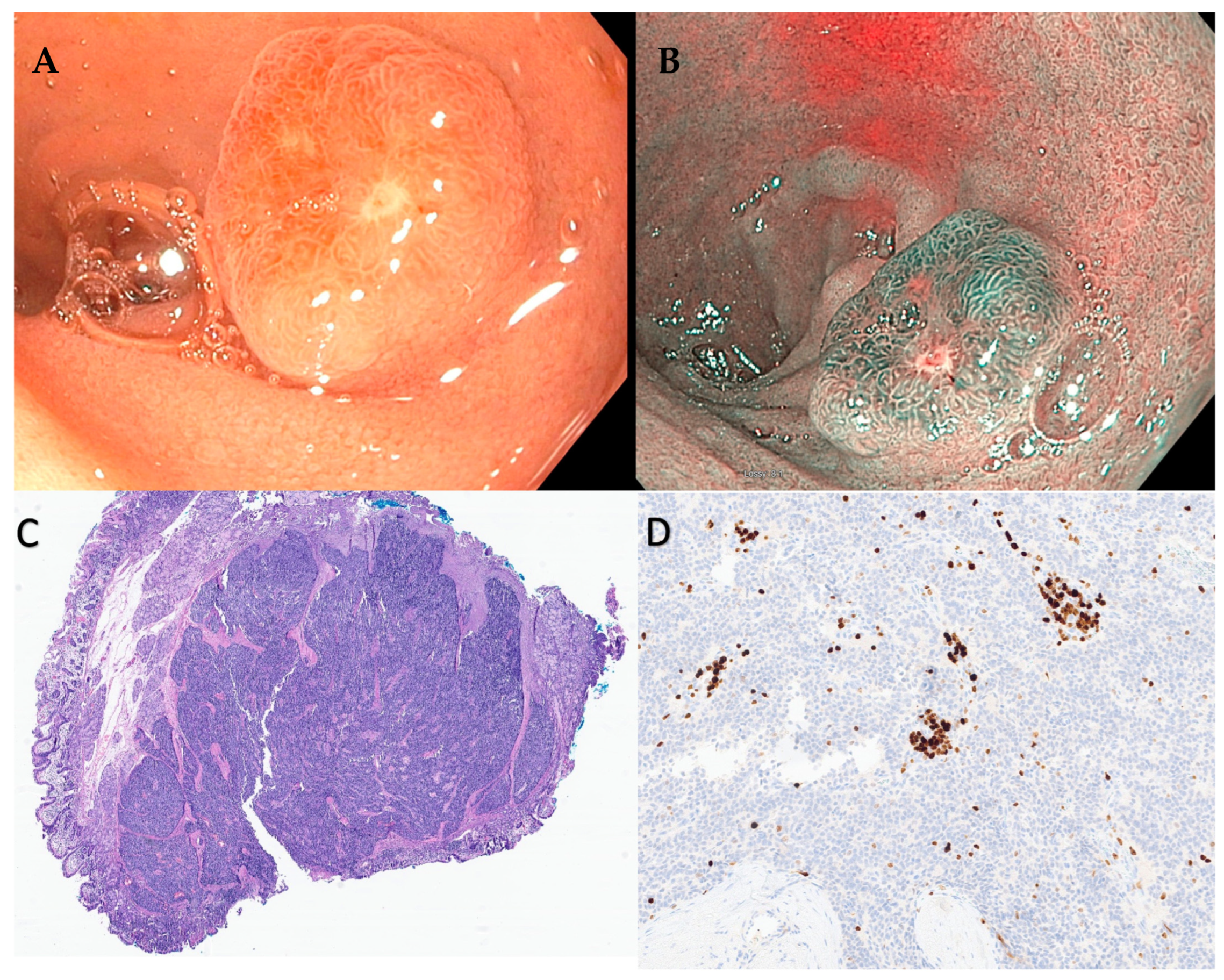
Based on a study using Surveillance, Epidemiology, and End Results (SEER) data from 1973 to 2012, the maximal increase in the incidence rate of NENs is for small intestinal NENs (0.8 per 100,000) with 38% of all gastrointestinal NENs found in the small intestine [3]. Small bowel NENs can be broadly conceptualized into two groups, those in the duodenum (d-NENs) and those in the rest of the small bowel, as these entities behave differently. One of the potential explanations for this is that duodenal tumors are easily identified using standard endoscopic techniques, while endoscopic access to the jejunum and ileum remains challenging. d-NENs also are identified incidentally on upper endoscopies that may be performed for other indications, which can result in earlier detection, while jejunum and ileum are not typically accessed on routine endoscopies. Duodenal NENs include gastrinomas, somatostatinomas, nonfunctional NENs, gangliocytic paragangliomas, and poorly differentiated neuroendocrine carcinomas [34]. Periampullary NENs and NENs of the ampulla of Vater are also duodenal, but they are often considered to be a separate class by many experts given their distinct histological, immunohistochemical, and growth behavior [34]. Most d-NENs are located in the first or second part of the duodenum, with about 20% occurring in the periampullary region [35]. Duodenal NENs can be asymptomatic or produce symptoms due to local infiltration, resulting in pain, gastrointestinal bleeding, and jaundice due to biliary obstruction or intestinal obstruction. Symptoms due to ectopic hormone release and classic carcinoid syndrome are rare (<10%) with duodenal compared to other small bowel NENs [34]. Neuroendocrine neoplasms (NENs) of the duodenum have also been categorized into ampullary and non-ampullary subtypes, depending on their anatomical location. Ampullary NENs, comprising 1/5th of duodenal NENs, exhibit distinct clinical characteristics when compared to non-ampullary NENs [36]. Ampullary is commonly observed in younger patients and frequently associated with Neurofibramatosis-1. Moreover, they are generally larger in size and tend to present with obstructive jaundice [37]. The ENETS 2016 consensus guidelines recommend surgical resection for ampullary and periampullary NETs [27]. However, for smaller ampullary NENs with a diameter less than 1 cm, endoscopic papillectomy has shown promise as a successful treatment option, particularly for low-grade tumors (G1), after ruling out muscular and bile duct invasion [38][39]. Notably, endoscopic papillectomy has also been documented for ampullary NENs larger than 10 mm [40][41]. Interestingly, recurrence after endoscopic papillectomy has not been observed so far, although further long-term studies are needed to validate this finding. Despite encouraging results of endoscopic papillectomy, surveillance intervals after endoscopic treatment for ampullary NENs remain to be established. For ampullary NENs greater than 1 cm in size, surgical resection with lymphadenectomy is generally recommended due to their higher likelihood of lymph node involvement [42]. This approach aims to achieve complete tumor removal and prevent potential metastatic spread. Similar to g-NENs, d-NENs also arise from the deep mucosal or submucosal layers and endoscopically appear as subepithelial lesions that are hemispherical or flatly elevated with reddish or yellowish hue on high-definition white light endoscopy (Figure 2 and Figure 3) [43].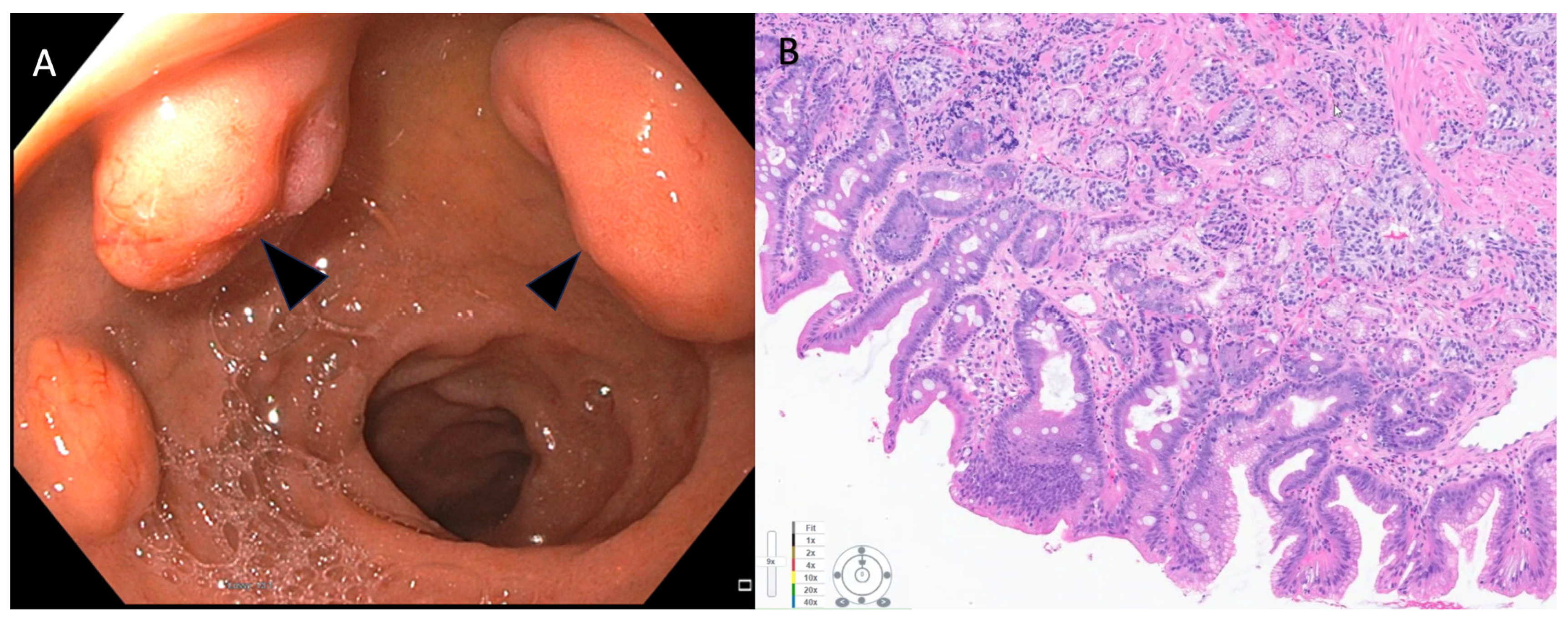
2.4. Appendix
Most appendiceal NENs are detected incidentally on appendectomy specimens. Seen in 0.3–0.9% of specimens, these are commonly located at the tip of the appendix [54]. However, when they develop at the appendiceal base and cause obstruction, they present as appendicitis in around 10% of the cases [55]. Careful endoscopic assessment of the appendix during regular colonoscopy can identify these lesions, which may be subtle and often mimic an inverted appendix. NCCB, NANETs, and ENTES recommend right hemicolectomy (RHC) for appendiceal NET ≥ 2 cm. Synchronous CRC must be ruled out by a complete colonoscopy before RHC [56][57][58]. Appendectomy is recommended for NET < 1 cm. Definitive guidelines regarding post-resection surveillance of NETs ≤ 2 cm are currently lacking. In NETs ≥ 2 cm, NCCN 2013 guidelines recommend a follow-up 3–12 months post-resection, followed by every 6–12 months up to 10 years, with imaging or lab markers [59].References
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002.
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868.
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342.
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188.
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99.
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 1–18.
- Lee, C.G.; Lim, Y.J.; Park, S.J.; Jang, B.I.; Choi, S.R.; Kim, J.K.; Kim, Y.T.; Cho, J.Y.; Yang, C.H.; Chun, H.J.; et al. The clinical features and treatment modality of esophageal neuroendocrine tumors: A multicenter study in Korea. BMC Cancer 2014, 14, 569.
- Deng, H.Y.; Ni, P.Z.; Wang, Y.C.; Wang, W.P.; Chen, L.Q. Neuroendocrine carcinoma of the esophagus: Clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J. Thorac. Dis. 2016, 8, 1250–1256.
- Giannetta, E.; Guarnotta, V.; Rota, F.; de Cicco, F.; Grillo, F.; Colao, A.; Faggiano, A. A rare rarity: Neuroendocrine tumor of the esophagus. Crit. Rev. Oncol. Hematol. 2019, 137, 92–107.
- Huang, Q.; Wu, H.; Nie, L.; Shi, J.; Lebenthal, A.; Chen, J.; Sun, Q.; Yang, J.; Huang, L.; Ye, Q. Primary high-grade neuroendocrine carcinoma of the esophagus: A clinicopathologic and immunohistochemical study of 42 resection cases. Am. J. Surg. Pathol. 2013, 37, 467–483.
- Kalemkerian, G.P. Staging and imaging of small cell lung cancer. Cancer Imaging 2012, 11, 253–258.
- Ilett, E.E.; Langer, S.W.; Olsen, I.H.; Federspiel, B.; Kjær, A.; Knigge, U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics 2015, 5, 119–176.
- Park, J.; Park, J.C.; Jo, J.H.; Kim, E.H.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Prospective comparative study of endoscopic ultrasonography-guided fine-needle biopsy and unroofing biopsy. Dig. Liver Dis. 2019, 51, 831–836.
- Zoundjiekpon, V.; Falt, P.; Fojtik, P.; Kundratova, E.; Mikolajek, O.; Hanousek, M.; Reiterova, K.; Ziak, D.; Bolek, M.; Tchibozo, A.; et al. Endosonography-Guided Fine-Needle Aspiration versus “Key-Hole Biopsy” in the diagnostics of upper gastrointestinal subepithelial tumors. A prospective randomized interventional study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2020, 164, 63–70.
- Sanaei, O.; Fernández-Esparrach, G.; De La Serna-Higuera, C.; Carrara, S.; Kumbhari, V.; El Zein, M.H.; Ismail, A.; Ginès, A.; Sendino, O.; Montenegro, A.; et al. EUS-guided 22-gauge fine needle biopsy versus single-incision with needle knife for the diagnosis of upper gastrointestinal subepithelial lesions: A randomized controlled trial. Endosc. Int. Open 2020, 8, E266–E273.
- Giri, S.; Afzalpurkar, S.; Angadi, S.; Sundaram, S. Mucosal incision-assisted biopsy versus endoscopic ultrasound-assisted tissue acquisition for subepithelial lesions: A systematic review and meta-analysis. Clin. Endosc. 2022, 55, 615–625.
- Lim, C.S.; Park, S.J.; Park, M.I.; Moon, W.; Kim, H.H.; Lee, J.S.; Kim, B.J.; Ku, D.Y. Successful endoscopic mucosal resection of a low esophageal carcinoid tumor. Clin. Endosc. 2013, 46, 576–578.
- Yagi, M.; Abe, Y.; Sasaki, Y.; Nomura, E.; Sato, T.; Iwano, D.; Yoshizawa, K.; Sakuta, K.; Kanno, N.; Nishise, S.; et al. Esophageal carcinoid tumor treated by endoscopic resection. Dig. Endosc. 2015, 27, 527–530.
- Goto, O.; Uraoka, T.; Horii, J.; Yahagi, N. Expanding indications for ESD: Submucosal disease (SMT/carcinoid tumors). Gastrointest. Endosc. Clin. N. Am. 2014, 24, 169–181.
- Li, Q.L.; Zhang, Y.Q.; Chen, W.F.; Xu, M.D.; Zhong, Y.S.; Ma, L.L.; Qin, W.Z.; Hu, J.W.; Cai, M.Y.; Yao, L.Q.; et al. Endoscopic submucosal dissection for foregut neuroendocrine tumors: An initial study. World J. Gastroenterol. 2012, 18, 5799–5806.
- Shim, C.S.; Jung, I.S. Endoscopic removal of submucosal tumors: Preprocedure diagnosis, technical options, and results. Endoscopy 2005, 37, 646–654.
- Gong, W.; Xiong, Y.; Zhi, F.; Liu, S.; Wang, A.; Jiang, B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy 2012, 44, 231–235.
- Deprez, P.H.; Moons, L.M.G.; O’Toole, D.; Gincul, R.; Seicean, A.; Pimentel-Nunes, P.; Fernández-Esparrach, G.; Polkowski, M.; Vieth, M.; Borbath, I.; et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 412–429.
- Ma, Z.; Cai, H.; Cui, Y. Progress in the treatment of esophageal neuroendocrine carcinoma. Tumor Biol. 2017, 39, 1010428317711313.
- Exarchou, K.; Howes, N.; Pritchard, D.M. Systematic review: Management of localised low-grade upper gastrointestinal neuroendocrine tumours. Aliment. Pharmacol. Ther. 2020, 51, 1247–1267.
- Sato, Y. Endoscopic diagnosis and management of type I neuroendocrine tumors. World J. Gastrointest. Endosc. 2015, 7, 346–353.
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124.
- Wang, R.; Zheng-Pywell, R.; Chen, H.A.; Bibb, J.A.; Chen, H.; Rose, J.B. Management of Gastrointestinal Neuroendocrine Tumors. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419884058.
- Chung, C.S.; Tsai, C.L.; Chu, Y.Y.; Chen, K.C.; Lin, J.C.; Chen, B.C.; Sun, W.C.; Yen, H.H.; Chen, C.Y.; Wu, I.C.; et al. Clinical features and outcomes of gastric neuroendocrine tumors after endoscopic diagnosis and treatment: A Digestive Endoscopy Society of Tawian (DEST). Medicine 2018, 97, e12101.
- Sato, Y.; Takeuchi, M.; Hashimoto, S.; Mizuno, K.; Kobayashi, M.; Iwafuchi, M.; Narisawa, R.; Aoyagi, Y. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology 2013, 60, 1524–1529.
- Jung, H.J.; Hong, S.J.; Han, J.P.; Kim, H.S.; Jeong, G.A.; Cho, G.S.; Kim, H.K.; Ko, B.M.; Lee, M.S. Long-term outcome of endoscopic and surgical resection for foregut neuroendocrine tumors. J. Dig. Dis. 2015, 16, 595–600.
- Meier, B.; Schmidt, A.; Glaser, N.; Meining, A.; Walter, B.; Wannhoff, A.; Riecken, B.; Caca, K. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: A prospective pilot study (RESET trial). Surg. Endosc. 2020, 34, 853–860.
- Kim, S.J.; Kim, T.U.; Choi, C.W.; Kim, H.W.; Park, S.B.; Ryu, D.G. Underwater endoscopic mucosal resection of upper gastrointestinal subepithelial tumors: A case series pilot study (with video). Medicine 2022, 101, e31072.
- Hoffmann, K.M.; Furukawa, M.; Jensen, R.T. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 675–697.
- Randle, R.W.; Ahmed, S.; Newman, N.A.; Clark, C.J. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: A population-based study. J. Gastrointest. Surg. 2014, 18, 354–362.
- Delle Fave, G.; Kwekkeboom, D.J.; Van Cutsem, E.; Rindi, G.; Kos-Kudla, B.; Knigge, U.; Sasano, H.; Tomassetti, P.; Salazar, R.; Ruszniewski, P. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 2012, 95, 74–87.
- Schmocker, R.K.; Wright, M.J.; Ding, D.; Javed, A.A.; Cameron, J.L.; Lafaro, K.; Burns, W.R.; He, J.; Wolfgang, C.L.; Burkhart, R.A. Duodenal, ampullary, and pancreatic neuroendocrine tumors: Oncologic outcomes are driven by tumor biology and tissue of origin. J. Surg. Oncol. 2021, 123, 416–424.
- Fukasawa, H.; Tounou, S.; Nabetani, M.; Michida, T. Endoscopic Resection of Ampullary Neuroendocrine Tumor. Intern. Med. 2017, 56, 499–503.
- Shimai, S.; Yamamoto, K.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Three Cases of Ampullary Neuroendocrine Tumor Treated by Endoscopic Papillectomy: A Case Report and Literature Review. Intern. Med. 2020, 59, 2369–2374.
- Odabasi, M.; Yildiz, K.M.; Cengiz, E.; Hasan, A.H.; Gunay, E.; Ozkan, E.; Aktekin, A.; Kaya, B.; Muftuoglu, T.M. Treatment of ampullary neuroendocrine tumor by endoscopic snare papillectomy. Am. J. Case Rep. 2013, 14, 439–443.
- Gincul, R.; Ponchon, T.; Napoleon, B.; Scoazec, J.Y.; Guillaud, O.; Saurin, J.C.; Ciocirlan, M.; Lepilliez, V.; Pioche, M.; Lefort, C.; et al. Endoscopic treatment of sporadic small duodenal and ampullary neuroendocrine tumors. Endoscopy 2016, 48, 979–986.
- Dogeas, E.; Cameron, J.L.; Wolfgang, C.L.; Hirose, K.; Hruban, R.H.; Makary, M.A.; Pawlik, T.A.; Choti, M.A. Duodenal and Ampullary Carcinoid Tumors: Size Predicts Necessity for Lymphadenectomy. J. Gastrointest. Surg. 2017, 21, 1262–1269.
- Sato, Y.; Hashimoto, S.; Mizuno, K.; Takeuchi, M.; Terai, S. Management of gastric and duodenal neuroendocrine tumors. World J. Gastroenterol. 2016, 22, 6817–6828.
- Kulke, M.H.; Shah, M.H.; Benson, A.B., 3rd; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Emerson, L.; Engstrom, P.F.; Fanta, P.; Giordano, T.; et al. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 78–108.
- Dasari, B.V.M.; Al-Shakhshir, S.; Pawlik, T.M.; Shah, T.; Marudanayagam, R.; Sutcliffe, R.P.; Mirza, D.F.; Muiesan, P.; Roberts, K.J.; Isaac, J. Outcomes of Surgical and Endoscopic Resection of Duodenal Neuroendocrine Tumours (NETs): A Systematic Review of the Literature. J. Gastrointest. Surg. 2018, 22, 1652–1658.
- Brito, H.P.; Torres, I.T.; Turke, K.C.; Parada, A.A.; Waisberg, J.; Botelho, R.V. Comparison of endoscopic resection techniques for duodenal neuroendocrine tumors: Systematic review. Endosc. Int. Open 2021, 9, E1214–E1221.
- Gopakumar, H.; Vohra, I.; Sharma, N.; Puli, S.R. Efficacy of self-assembling peptide in mitigating delayed bleeding after advanced endoscopic resection of gastrointestinal lesions. A meta-analysis. Endosc. Int. Open 2023, 11, E553–E560.
- Hoteya, S.; Kaise, M.; Iizuka, T.; Ogawa, O.; Mitani, T.; Matsui, A.; Kikuchi, D.; Furuhata, T.; Yamashita, S.; Yamada, A.; et al. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: Analysis of risk factors. Dig. Endosc. 2015, 27, 323–330.
- Mori, H.; Shintaro, F.; Kobara, H.; Nishiyama, N.; Rafiq, K.; Kobayashi, M.; Nakatsu, T.; Miichi, N.; Suzuki, Y.; Masaki, T. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig. Endosc. 2013, 25, 459–461.
- Tsujimoto, H.; Ichikura, T.; Nagao, S.; Sato, T.; Ono, S.; Aiko, S.; Hiraki, S.; Yaguchi, Y.; Sakamoto, N.; Tanimizu, T.; et al. Minimally invasive surgery for resection of duodenal carcinoid tumors: Endoscopic full-thickness resection under laparoscopic observation. Surg. Endosc. 2010, 24, 471–475.
- Wang, S.C.; Parekh, J.R.; Zuraek, M.B.; Venook, A.P.; Bergsland, E.K.; Warren, R.S.; Nakakura, E.K. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch. Surg. 2010, 145, 276–280.
- Furnari, M.; Buda, A.; Delconte, G.; Citterio, D.; Voiosu, T.; Ballardini, G.; Cavallaro, F.; Savarino, E.; Mazzaferro, V.; Meroni, E. The role of wireless capsule endoscopy (WCE) in the detection of occult primary neuroendocrine tumors. J. Gastrointest. Liver Dis. 2017, 26, 151–156.
- Gangi, A.; Siegel, E.; Barmparas, G.; Lo, S.; Jamil, L.H.; Hendifar, A.; Nissen, N.N.; Wolin, E.M.; Amersi, F. Multifocality in Small Bowel Neuroendocrine Tumors. J. Gastrointest. Surg. 2018, 22, 303–309.
- Roggo, A.; Wood, W.C.; Ottinger, L.W. Carcinoid tumors of the appendix. Ann. Surg. 1993, 217, 385–390.
- Moertel, C.G.; Dockerty, M.B.; Judd, E.S. Carcinoid tumors of the vermiform appendix. Cancer 1968, 21, 270–278.
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010, 39, 753–766.
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176.
- Heller, D.R.; Jean, R.A.; Luo, J.; Kurbatov, V.; Grisotti, G.; Jacobs, D.; Chiu, A.S.; Zhang, Y.; Khan, S.A. Practice Patterns and Guideline Non-Adherence in Surgical Management of Appendiceal Carcinoid Tumors. J. Am. Coll. Surg. 2019, 228, 839–851.
- NCCN Guidelines. Available online: https://www.nccn,org/preofessionals/phyiciab_gls/pdf/neuroendocrine.pdf (accessed on 23 April 2023).
