Retinal prosthetics show promise in restoring vision for individuals with retinal diseases. Technological advancements have led to various implant designs, expanding possibilities for enhancing visual acuity. A resviearchw explores pre-clinical and clinical studies, engineering aspects, safety, adverse events, and rehabilitation programs. The debate over alternative therapies is addressed, comparing retinal prostheses to cell-based and gene-based therapies and optogenetics. Advancements in wireless technology and artificial intelligence are discussed. The retinal structure and function are outlined, emphasizing the importance of different retinal layers. Retinal physiology poses challenges for prosthetic devices, requiring selective stimulation for optimal outcomes. Visual prosthetics aim to restore vision by replicating retinal ganglion cells' electrical signals. Two mechanisms are used: (1) external camera systems process images to deliver electrical impulses via an implanted microelectrode array, as seen in the ARGUS II prosthetic, and (2) photodiode arrays directly convert light energy to electrical signals.
- Retinal prosthetics
- Photoreceptors
- Implant designs
- Visual acuity
- Ophthalmology
- Retinal disease
- Retinal anatomy
- Electrical stimulation
1. Overview of Retinal Prostheses
Overview of Retinal Prostheses
Recent advancements in retinal prosthetics have garnered attention for their potential to restore vision in individuals with retinal diseases that cause photoreceptor loss, like age-related macular degeneration and retinitis pigmentosa. These conditions severely impact patients' quality of life and daily activities. Retinal prostheses, also known as bionic eyes, stimulate remaining retinal cells to create visual sensations.
Technological progress in retinal prosthetics includes various implant designs, such as epiretinal, subretinal, and suprachoroidal sensors, expanding possibilities for improving visual acuity and device functionality.
This entry includes data from pre-clinical and clinical studies conducted between 2017 and 2023, exploring retinal anatomy, physiology, pathology, and principles of electronic retinal prostheses. It covers engineering aspects like electrode alignment, size and material, charge density, resolution limits, spatial selectivity, and bidirectional closed-loop systems. Clinical aspects, including safety, adverse events, visual function, outcomes, and the importance of rehabilitation programs, are discussed.
The resviearchw addresses the ongoing debate on implantable retinal devices versus emerging cell-based and gene-based therapies and optogenetics, providing a balanced perspective on their advantages and limitations. Recent advancements in retinal prosthetics are outlined, emphasizing engineering progress and future prospects. The integration of wireless technology and artificial intelligence for improved signal processing and transmission is also highlighted.
2. Overview of Retinal Structure and Function
Overview of Retinal Structure and Function
The retina, originating from the inner and outer layers of the optic cup, is a thin, transparent structure comprising 10 distinct layers crucial for visual processing. In cross section, these layers can be identified as follows (Figure 1).
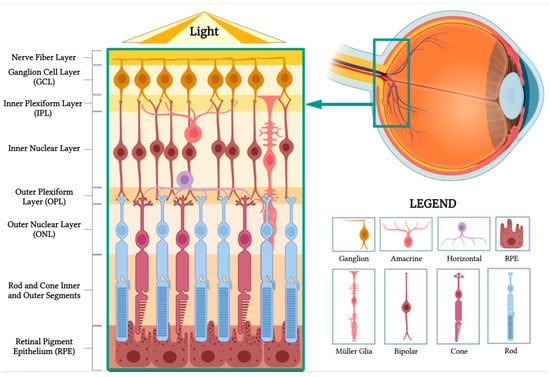
Figure 1. Retinal anatomy. The illustration highlights the different layers of the retina and its main cell types. (BioRender, https://app.biorender.com/, accessed on 16 February 2023).
- Nerve fiber layer: Contains axons of retinal ganglion cells, forming the optic nerve.
- Ganglion cell layer: Composed of the cell bodies of retinal ganglion cells, transmitting visual information to the brain.
- Inner plexiform layer: Synapses between bipolar and ganglion cells facilitate signal processing and integration.
- Inner nuclear layer: Houses the cell bodies of bipolar, horizontal, and amacrine cells involved in processing and transmitting visual information.
- Outer plexiform layer: Contains synapses between photoreceptors, bipolar cells, and horizontal cells, enabling initial visual signal processing.
- Outer nuclear layer: Comprises the cell bodies of rod and cone photoreceptors responsible for capturing and converting light into neural signals.
- Rod and cone inner and outer segments: Photoreceptor segments that contain vital cellular components and photopigment-rich discs, initiating phototransduction.
- Retinal pigment epithelium (RPE): The outermost layer beneath photoreceptors, with functions including light absorption, photopigment recycling, and nutrient transport.
Horizontal cells form synapses with rod spherules and cone pedicles, while vertically oriented bipolar cells synapse with rod or cone synaptic bodies. Bipolar cell axons make synaptic contact with ganglion cells and amacrine cells in the inner plexiform layer. Ganglion cell axons constitute the nerve fiber layer and optic nerve, comprising over one million optic nerve fibers[1].
3. Overview of Retinal Physiology and Pathology
Overview of Retinal Physiology and Pathology
Retinal prostheses aim to restore vision by replacing the function of degenerate photoreceptors. In the normal eye, photoreceptors in the outer retina trigger the phototransduction cascade, generating neuronal signals upon light stimulation. These signals are processed by retinal neurons before reaching retinal ganglion cells (RGCs) in the inner layers. RGC axons form the optic nerve, transmitting light-evoked signals to the visual cortex. However, in retinal dystrophies like retinitis pigmentosa, outer retinal photoreceptors are gradually lost, leading to progressive visual impairment, while inner retinal layers, including RGCs and bipolar cells, are partially preserved[2].
Theoretically, retinal prostheses could restore vision by receiving and processing light, transmitting electrical impulses to the inner retinal layers for visual function. However, the retina's complex physiology presents challenges, as prosthetic devices need to replicate intricate retinal processing. Current stimulation methods lack selectivity, simultaneously activating multiple cell types. Developing more specific stimulation is crucial to optimize device resolution and improve patient outcomes.
4. Principles of Electronic Retinal Prostheses
The field of visual prosthetics aims to restore vision to the blind by replicating the complex electrical signals of retinal ganglion cells. The ultimate goal is to create vision comparable to that of a healthy retina, where retinal prostheses receive a light stimulus, convert it to an electric pulse, and deliver it to the retina through a microelectrode array. The electric signal stimulates the remaining structures in the retina that can convey signals to the optic nerve for interpretation[3].
Two main mechanisms provide electrical stimulation to retinal cells in prostheses[4]. The first mechanism involves external light detection and internal impulse delivery. An intraocularly implanted microelectrode array depends on an external camera system, typically mounted on glasses, to capture the surrounding image. The image is processed and converted into electrical impulses, which are then sent to a periocular implant (Figure 2). Through cables, the periocular implant delivers the electrical signal to the implanted microelectrode array, which conveys it to the retinal cells. The ARGUS II prosthetic exemplifies this system (Figure 2 and Figure 3)[5].
The second mechanism utilizes a photodiode electrode array that directly receives and transduces light energy into an electrical signal (Figure 4)[6]. These photodiode-based systems aim to replace the lost photoreceptors of the retina and often function without processing units or external cameras. In some cases, external components, including power sources, amplify the light waves to generate suitable impulses[7]. Prostheses employing this mechanism are activated directly by light and rely on photovoltaic processes, such as voltage drop creation, to stimulate target cells[8]. Photodiode systems offer advantages: (1) they eliminate the need for implanted cables due to their photovoltaic process, simplifying surgical procedures compared to cable-dependent implants, and (2) they directly transduce light stimuli into electrical signals, often not requiring an external camera. This preservation of the link between eye movements and visual stimuli is beneficial[9].
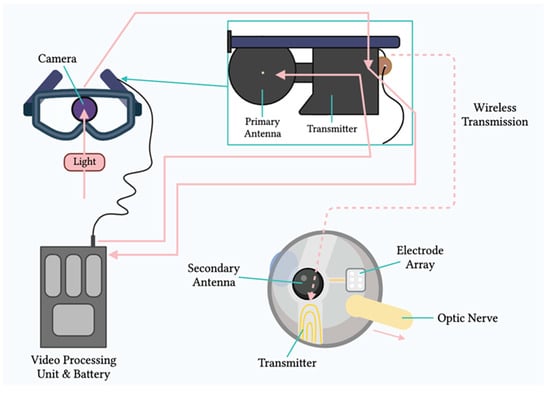
Figure 2. Components of the ARGUS II system. Schematic representation of the main components of the ARGUS II retinal prosthesis system, including the camera, video processing unit, and electrode array.
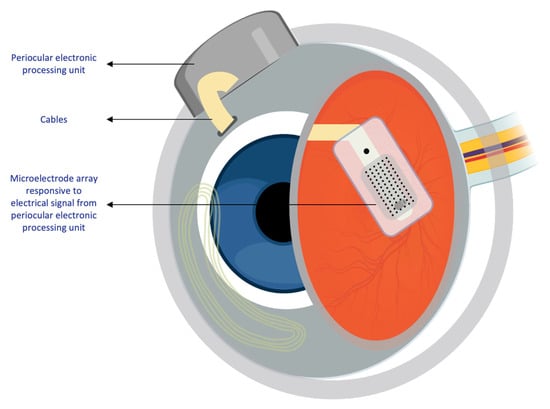
Figure 3. Intraocular components of the ARGUS II system. Diagram showing the internal components of the ARGUS II retinal prosthesis system that are implanted within the eye.
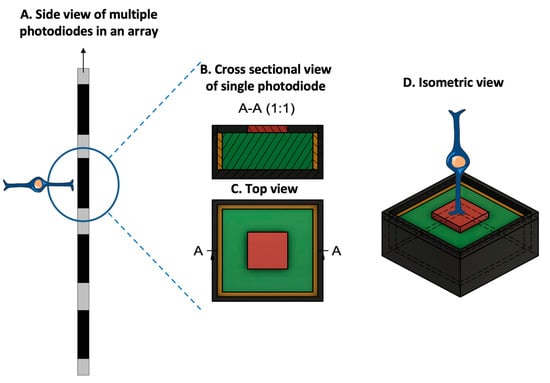
Figure 4. Prototypical design configuration of a photodiode, consisting of an inner electrode (red), a photodiode (green), an insulating layer (yellow), and an outer ground electrode (dark gray). (A. Side view of multiple photodiodes in an array. B. Cross-sectional view of the photodiode at position A-A, at the location specified in the top view. C. Top-orientation view of photodiode. D. Isometric view of the photodiode with a bipolar cell in close relation to the inner electrode. Due to the voltage drop between the inner and outer electrodes, the photodiode generates an electric field that can be used for cell stimulation. (Figure 4 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
Regardless of the mechanism, both systems, whether utilizing internal non-light-sensitive electrodes or photodiodes, need to meet common engineering design criteria. In this entry, the term "microelectrode array" (MEA) encompasses both types of arrays as they employ electrodes to convey electric impulses to retinal cells[10].
References
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Kolb, H.; Fernandez, E.; Nelson, R. Anatomy and Physiology of the Retina; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995.
- Abbasi, B.; Rizzo, J.F. Advances in Neuroscience, Not Devices, Will Determine the Effectiveness of Visual Prostheses. Semin. Ophthalmol. 2021, 36, 168–175.
- Mirochnik, R.M.; Pezaris, J.S. Contemporary Approaches to Visual Prostheses. Mil. Med. Res. 2019, 6, 19.
- Ghezzi, D. Translation of a Photovoltaic Retinal Prosthesis. Nat. Biomed. Eng. 2020, 4, 137–138.
- Cehajic-Kapetanovic, J.; Singh, M.S.; Zrenner, E.; MacLaren, R.E. Bioengineering Strategies for Restoring Vision. Nat. Biomed. Eng. 2022, 7, 387–404.
- Zrenner, E.; Bartz-Schmidt, K.U.; Benav, H.; Besch, D.; Bruckmann, A.; Gabel, V.-P.; Gekeler, F.; Greppmaier, U.; Harscher, A.; Kibbel, S.; et al. Subretinal Electronic Chips Allow Blind Patients to Read Letters and Combine Them to Words. Proc. Biol. Sci. 2011, 278, 1489–1497.
- Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L.; Kamins, T.I.; Galambos, L.; Smith, R.; Harris, J.S.; Sher, A.; et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nat. Photonics 2012, 6, 391–397.
- Huang, T.W.; Kamins, T.I.; Chen, Z.C.; Wang, B.-Y.; Bhuckory, M.; Galambos, L.; Ho, E.; Ling, T.; Afshar, S.; Shin, A.; et al. Vertical-Junction Photodiodes for Smaller Pixels in Retinal Prostheses. J. Neural Eng. 2021, 18, 036015.
- Watterson, W.J.; Montgomery, R.D.; Taylor, R.P. Modeling the Improved Visual Acuity Using Photodiode Based Retinal Implants Featuring Fractal Electrodes. Front. Neurosci. 2018, 12, 277.
