Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Maksim Kaimonov and Version 2 by Sirius Huang.
Oxides of sodium, calcium, silicon, and phosphorus form the Na2O–CaO–SiO2–P2O5 system, of which the most famous material—Bioglass 45S5—was developed in 1969 by Professor Larry Hench. In the middle of the 1980s, Bioglass 45S5 was introduced to the market, and it stimulated many research groups to an intensive investigation of bioactive glasses and their further applications.
- Bioglass 45S5
- bioactive glass–ceramics
- calcium phosphates
- Na2O–CaO–SiO2–P2O5 system
- composites
1. The Na2O–CaO–SiO2–P2O5 System
The original idea of Larry Hench was to combine the elements that a human body is replete with in proportions that facilitate the rapid release of alkalis from the glass surface in aqueous solutions, followed by the formation of layers rich in calcium and phosphorus. Thus, the Na2O–CaO–SiO2 system was chosen as a base, to which phosphorus oxide (P2O5) was also added in small amounts (6 wt.%) since it was believed that its presence in glass or ceramics was critical to the bioactivity of the material at the time [1][24]. However, the development of modern approaches to obtaining an “ideal” biomaterial has shown that phosphate-free glasses and ceramics also have bioactivity [2][3][25,26]. For instance, calcium silicate (CaSiO3) bioceramics have significantly greater osteoinductive capacity, and it has been observed both in vitro and in vivo compared with tricalcium phosphate [2][25]. Calcium silicate extract promoted macrophage polarization, thus reducing the host-to-material inflammatory response. In addition, after stimulation by a macrophage-conditioned medium pretreated by calcium silicate extracts, the osteogenic differentiation of bone marrow stromal cells (BMSCs) was greatly enhanced by macrophage-derived oncostatin M [2][25]. Nevertheless, phosphorus oxide plays a specific role in the composition of the material (it accelerates the formation of hydroxyapatite and hydroxyl carbonate apatite layers on the surface of the material), so its presence is desirable in the final product. Thus, the Na2O–CaO–SiO2–P2O5 system can be schematically presented in a Na2O–CaO–SiO2 phase diagram (Figure 1) with the fixed mass value of P2O5 equal to 6 wt.% [4][17].
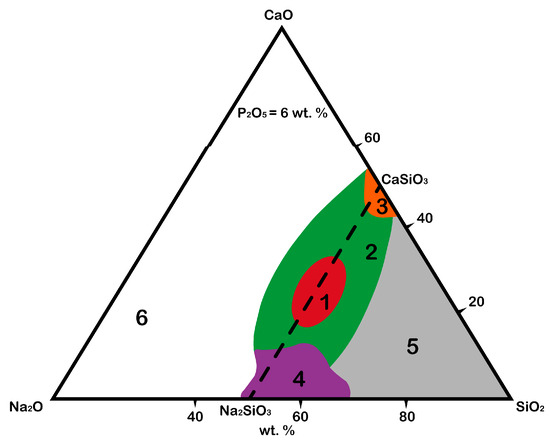
Figure 1.
Schematic of bioactive regions in the Na
2
O–CaO–SiO
2
system with fixed amount of P
2
O
5
.
The diagram shows the areas responsible for the possibility of the formation of chemical bonds between material and native bone tissue after the integration of the material into a body [5][6][19,27]:
-
The red area (1) is the area of biological activity of class A, in which the corresponding biologically active glasses are osteoproductive (bind to bone and soft tissue, activate genes). The formation of the HAp layer is observed several hours after integration into a body.
It was proved that bioglass grade 45S5 (Bioglass®) forms such a strong bond with a bone that the implant cannot be removed without destroying it [7][28]. This effect is observed due to the saturation of the bone tissue defect with calcium and silicon ions, which stimulate osteogenic cells to form a bone matrix; -
The green area (2) is the area of biological activity of class B, in which the corresponding biologically active glasses are osteoconductive (bind only to bone tissue). The formation of the HAp layer is observed from 24 to 96 h after integration into a body;
-
The orange area (3) is the area of biological activity in which the formation of Cerabone bioglass ceramics takes place, consisting of apatite (Ca10(PO4)6(OH1F2)) and wollastonite (CaO·SiO2) crystals as well as a residual SiO2 glassy matrix [5][19]; however, unlike in other areas, the P2O5 content may vary;
-
The purple area (4) is where the corresponding biologically active glasses are fully resorbed in the body after 10 to 30 days with a minimal restoration of damaged bone tissue;
-
The gray area (5) is where the corresponding glasses do not form bonds even with bone and behave like a bioinert material;
-
The white area (6) is where glass formation is not observed.
Traditionally, the Na2O–CaO–SiO2 system has been used in the production technology of soda lime silicate glasses (windows, glass, bottles, containers, and so on) where Na2O, CaO, and SiO2 are the main components. The differences between bioactive glasses and industrial glasses are [5][19]: (1) SiO2 content less than 60 mol%, (2) high Na2O and high CaO content, and (3) high CaO/P2O5 ratio. These compositional features make the surface highly reactive when it is exposed to an aqueous medium.
1.1. Benefits and Drawbacks of Bioactive Glass 45S5
In the middle of the 1980s, Bioglass 45S5 was introduced to the market, and it stimulated many research groups to an intensive investigation of bioactive glasses and their further applications. Today, more than 1000 articles have been published [1][24] dedicated to studying the physicochemical properties of Bioglass 45S5 and resembling materials, its bioactivity, bioresorbability, and the limits of its applicability. A massive array of data makes it possible to highlight both the benefits and drawbacks of bioglass compositions in the Na2O–CaO–SiO2–P2O5 system.
The main benefit of bioglass materials in the Na2O–CaO–SiO2–P2O5 system is that their dissolution products enhance the expression of genes that control osteogenesis [8][9][29,30]; thus, they provide a higher rate of bone formation in comparison with other inorganic bioceramic materials, such as hydroxyapatite [10][31]. One of the explanations is the presence in most bioglasses of sodium oxide (Na2O) in sufficiently large quantities to create an alkaline environment, providing an antibacterial effect and promoting early dissolution of the implant material with the formation of layers rich in HAp, HCA, and silicon dioxide [11][12][18,32]. The studies [13][14][33,34] demonstrated that Bioglass 45S5 is efficient against Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli.
The drawbacks of bioglass materials in the Na2O–CaO–SiO2–P2O5 system are again associated with a high alkali content, namely: a relatively fast rate of dissolution and resorption [1][15][24,35], which negatively affects the balance of natural remodeling of bone tissue and leads to the formation of a gap between the tissue and the implant material [16][36]; low mechanical strength of scaffold structures; and significant cytotoxic effect caused by high doses of sodium leached into the culture medium [17][37].
One of the currently used methods for increasing the mechanical strength of bioglass materials is devitrification, as a result of which crystalline phases are formed in the material.
1.2. Methods for Obtaining Crystal Phases
Devitrification of bioglass is a simple method for obtaining crystal phases and includes glass preparation, grinding obtained glass to a size of 60–150 microns, and the firing of glass powder at temperatures no higher than 1050 °C with an exposure of 1.5–2 h. This approach is not universal and has a generalized character according to the literature data. Additional components can be added to a glass charge to form crystal phases of a given composition while an individual heat treatment mode is selected.
Bioactive glasses can be obtained by two methods (Table 1): traditional quenching from a melt and sol–gel.
Table 1.
Methods for obtaining bioglass in the Na
2
O–CaO–SiO
2
–P
2
O
5
system.
| Melt–Quench [18][19][20][21][22] | Melt–Quench [38,39,40,41,42] | Sol–Gel [23][24][25] | Sol–Gel [43,44,45] | |
|---|---|---|---|---|
| Initial reagents |
Sodium carbonate (Na2CO3); calcium carbonate (CaCO3) or calcium oxide (CaO); phosphorus pentoxide (P2O5); silicon dioxide (SiO2). |
Tetraethylorthosilicate (TEOS) or tetramethylortosilicate (TMOS); triethyl phosphate (TEP); calcium nitrate tetrahydrate (Ca(NO3)2·4H2O); sodium nitrate (NaNO3). |
||
| Steps |
|
|
||
| Advantages | Traditional approach; simple. |
Higher purity and homogeneity; a wide range of compositions; possibility of obtaining silica gel at room temperature. |
||
| Disadvantages | Energy intensive; requires complete homogenization of the melt; may lead to contamination from the chemical substances; no possibility to fabricate porous scaffolds. |
Dependence on pH; monoliths of bioactive glass (d > 1 cm) have cracks due to the shrinkage that occurs during drying and the evaporation of the liquid by-products of the condensation reaction; alkoxides are not suitable for large-scale production. |
Nevertheless, the approach to obtaining crystal phases has difficulties related to forming stoichiometric compounds and repeatability limits. The phase compound of such materials can either coincide or differ from the composition of the initial amorphous phase [26][27][46,47]. Thus, the formation of both one phase and many crystalline phases of different chemical compositions with preservation of the bioactivity of some can occur during devitrification.
On the other hand, spark plasma sintering (SPS) may be used for producing fully dense and completely amorphous Bioglass 45S5 specimens at temperatures as low as 500–550 °C and crystallized Bioglass 45S5 specimens with Na2CaSi2O6 phase composition at 600 °C. It has been confirmed the even higher bioactivity of Na2CaSi2O6 is obtained by SPS compared to by amorphous Bioglass 45S5 also produced by SPS at 500–550 °C or conventionally sintered Bioglass 45S5 (crystallized by high-temperature treatment at 1050 °C) [28][48].
2. The Na2SiO3–CaSiO3 System
The area of biological activity of classes A and B, where the corresponding biologically active glasses are osteoproductive and osteoconductive, respectively, is located on the line of the Na2SiO3–CaSiO3 phases (Figure 1). Therefore, it can be assumed that the crystallization of the compounds lying on the line of these phases can form biologically active crystalline phases (Figure 2).
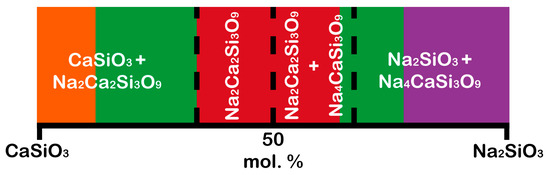
Figure 2. Schematic of bioactive regions in the Na2SiO3–CaSiO3 system. The red color is the area of biological activity of class A; the green color is the area of biological activity of class B; the orange color is the area of obtaining the Cerabone bioglass ceramics; the purple color is bioglasses provide a minimal restoration of damaged bone tissue due to their high resorption. (Color description is related to Figure 1).
The pseudo-binary Na2SiO3–CaSiO3 system includes five crystalline phases [29][49]: CaSiO3 (wollastonite (β-CaSiO3), JCPDS 43-1460, and pseudowollastonite (α-CaSiO3), JCPDS 31-300), Na2O·CaO·2SiO2 (112, JCPDS 77-2189), Na2O·2CaO·3SiO2 (123, combeite, JCPDS 75-1686), 2Na2O·CaO·3SiO2 (213, JCPDS 37-0282), and Na2SiO3 (JCPDS 16-0818).
Comparison between glasses (CaSiO3, 112, 123, 213, and Na2SiO3) and crystalline phases (CaSiO3, 112, 123, 213, and Na2SiO3) demonstrated that crystalline phases did not inhibit the development of the HAp and HCA layers due to P2O5-free compositions possessing the ability to form these layers because phosphorus ions P5+ can be obtained from human blood. Nevertheless, crystalline phases affected their formation rate [12][32]. In other words, the formation of the HAp and HCA layers on the crystalline phases was observed to be 2–4 times slower than on the corresponding glasses while maintaining bioactivity [27][30][47,50]. Thus, by selecting the composition of a biomaterial, it is possible to control the rate of its resorption in the body.
Speaking of calcium silicate (CaSiO3) ceramics, it is worth noting that CaSiO3 has excellent biocompatibility and mechanical properties compared to hydroxyapatite, as well as high bioactivity and a faster dissolution rate compared with calcium phosphate materials [31][32][33][51,52,53]. In addition, a relatively extensive chemical composition range of silicate ceramics allows for optimization of the phase composition to improve the mechanical properties of bioactive ceramics in contrast to phosphate ceramics [34][54].
The addition of sodium silicate leads to sodium–calcium silicates formation, which has been studied in works [35][36][37][55,56,57]. For instance, Durgalakshmi et al. [35][55] estimated the biocompatibility of bioglass with phase composition Na4CaSi3O9 and Na2Ca3Si6O16 from MTT assay, where the absorbance of the MTT assay for 3T3 cell lines was observed at 570 nm for all the samples after three days. The result showed that all materials had 50% to 60% cell viability for fibroblast cell lines concerning osteoblast cell lines in bone reconstruction (the percentage of fibroblast formation must be less but not zero). The authors demonstrated that crystallized bioglass with phase composition Na4CaSi3O9 and Na2Ca3Si6O16 is a prospect in tissue engineering applications.
Moreover, Lin et al. [36][56] noted several aspects, for example, the comparable elastic moduli of the 45S5-derived crystal phase (Na2CaSi2O6) with hydroxyapatite and the ability to develop an HCA layer in simulated body fluid (SBF); in comparison with the Na2CaSi2O6, 45S5 glass possessed a higher in vivo bioactivity index due to it being amorphous. It indicates that the 45S5-derived crystal phase (Na2CaSi2O6) is better suited for use as a substitute for bone than its glass.
In addition, work [37][57] demonstrated the bioactivity of Na2Ca2Si3O9 by soaking Na2Ca2Si3O9 disks in SBF. The results in vitro showed that hydroxyapatite (HAp) formed on the surface of Na2Ca2Si3O9 samples after soaking for 1 day, which indicated the good bioactivity of Na2Ca2Si3O9.
According to the literature data, the main crystal phase formed during the devitrification of pure Bioglass 45S5 glasses has not been established precisely. Some authors indicated the formation of the Na2Ca2Si3O9 phase [38][39][40][58,59,60]; others pointed at the Na6Ca3Si6O18 phase formation [24][26][44,46]; and there are works in which Na4Ca4Si6O18 was determined as the crystalline phase after glass devitrification [41][42][23,61]. The current uncertainty can be explained by the close lattice parameters of all three compounds, Na2Ca2Si3O9 (hexagonal, P31 (152), a = 10.464, c = 13.176) [43][62], Na4Ca4Si6O18 (hexagonal, P32 (152), a = 10.464, c = 13.168) [44][63], and Na6Ca3Si6O18 (rhombohedral, R-3m (166), a = 10.500, c = 13.184) [45][64], as well as by the isostructurality of the Na6Ca3Si6O18 and Na4Ca4Si6O18 phases and the high-temperature form Na2Ca2Si3O9. Thus, clear identification of the main crystalline phase of ceramic from sintered bioglass is impossible due to the close structural similarity of these phases. Nevertheless, work is underway to study crystal phases in glass–ceramics. For example, the authors of [26][46] gave several arguments in favor of the Na6Ca3Si6O18 phase formation after the devitrification of Bioglass 45S5. Sodium–calcium silicate phase determination is a challenging task, and it becomes more sophisticated when composites are obtained.
