Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Patricia Herrera and Version 2 by Fanny Huang.
Dialysis treatment has improved the survival of patients with kidney failure. However, the hospitalization and mortality rates remain alarmingly high, primarily due to incomplete uremic toxin elimination. High-volume hemodiafiltration (HDF) has emerged as a promising approach that significantly improves patient outcomes by effectively eliminating medium and large uremic toxins, which explains its increasing adoption, particularly in Europe and Japan.
- hemodiafiltration
- dialysis
- uremic toxins
- high-volume hemodiafiltration
1. Introduction
The kidney can eliminate small molecules, as well as peptides with molecular weights between 10 and 30 kDa. In end-stage renal failure, dialysis has alleviated the classic symptoms of uremia by eliminating small molecules. However, it is possible to observe the persistence of “residual” signs and symptoms due to insufficient removal of larger molecules, resulting in physical and cognitive limitations, metabolic effects, and cellular dysfunction [1][22].
The conventional HD prescription allows the removal of approximately two thirds of the total body urea content during each treatment. Along with this, most of the small uremic toxins similar to urea, which can easily move via diffusion, are removed. The HEMO study showed that the results do not improve with an increase in fractional urea elimination above the current standard [2][23]. This is because the elimination of many solutes is limited due to their large molecular size, their binding to proteins, or their retention in body compartments. Therefore, plasma levels of these solutes remain much higher than normal urea levels in patients undergoing conventional dialysis [1][22].
Frequent complications, such as cardiovascular disease, anemia, bone disorders, and neuropathy, are still observed in patients with adequate Kt/V and have been correlated with the difficulty in removing uremic toxins in the molecular range of 5000 to 50,000 daltons [3][24].
The spectrum of medium-weight molecules includes β2M, used as an indicator of medium-toxin clearance and correlated as a risk factor for mortality; fibroblast growth factor 23 (FGF-23) along with parathyroid hormone (PTH), osteocalcin and osteoprotegerin, implicated in alterations of bone metabolism; indoxyl sulfate and p-cresol sulfate, which mainly bind to albumin and have been correlated with an increased risk of cardiovascular events; leptin, for its involvement in reducing appetite in dialysis patients; and free chains of immunoglobulins, hepcidin and homocysteine, which have also been identified as toxic inflammatory molecules in uremic patients [4][25].
Many studies of high-volume HDF have demonstrated superiority for the removal of these toxins compared with HF-HD [5][6][26,27], and the maximum benefit is achieved by exceeding 24 L of convective volume in a 4-h session, which is particularly important for β2M [7][8][8,28]. On the other hand, protein-bound toxins such as p-cresylglucuronide, hippuric acid, indole acetic acid, indoxyl sulfate, and p-cresol sulfate could be removed more efficiently by HDF [9][29].
2. Phosphatemia
Studies with high-volume HDF are consistent in showing greater phosphorus removal compared to HD [10][11][30,31]. However, this could have a modest effect on predialysis phosphatemia, with an estimated drop of less than 15%, since patients who switch to HDF additionally improve their appetite and ingest greater amounts of protein and phosphorus [12][13][14][15,16,32], but also because phosphorus does not follow the same removal kinetics as urea, rapidly reaching a plateau phase after which phosphatemia levels do not fall any further [15][16][33,34], and a plasma rebound is observed after the end of the dialysis session, which also occurs in HDF [17][35]. This has been explained by kinetics of phosphorus body distribution that involve four compartments and a series of regulatory mechanisms that hinder the movement of phosphorus in an environment of abrupt changes in phosphatemia in a compensatory manner (Figure 1) [18][36]. Thus, it has been demonstrated with magnetic resonance spectroscopy that the phosphorus removed during dialysis mainly comes from the intracellular space and more specifically from the ATP deposits within the mitochondria [19][37]. These kinetics allow us to understand that the most efficient way to remove phosphorus is by prolonging the dialysis time [20][21][38,39] or through high-volume HDF for more than 4 h [16][22][34,40].
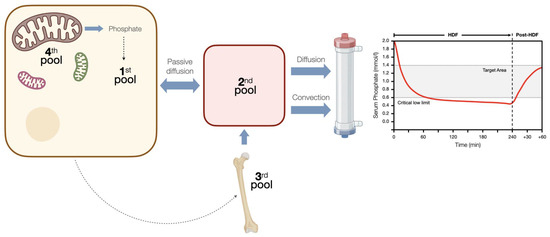
Figure 1. Phosphorus kinetics during hemodiafiltration. During hemodiafiltration, the levels of phosphate in the blood rapidly reach a plateau, below which they do not decrease. After therapy, there is a rebound effect that persists for over an hour. This can be explained by a four-compartment system with controlled kinetics, which responds to changes in the concentration of intracellular phosphate. Initially, there is a dynamic equilibrium established between the intracellular compartment (1st pool) and the extracellular compartment (2nd pool). Once the serum phosphate levels reach a critically low limit, the intravascular space receives additional phosphorus, potentially originating from a phosphate reservoir that has not yet been incorporated into the bone matrix (3rd pool). It is plausible that changes in the concentration of phosphate inside the cells trigger this control mechanism. Finally, when intracellular phosphate levels drop below a critical threshold (<0.97 mmol/L), there is an immediate release of phosphate from a fourth pool into the intracellular space. This 4th pool is believed to consist of phosphate derived from mitochondrial adenosine triphosphate (ATP). This mechanism serves to protect the intracellular environment from dangerously low concentrations of phosphate.
3. Oxidative Stress and Inflammation
Uremia is marked by heightened oxidative stress (OS) due to increased prooxidant molecules, poor oxidative product clearance, and deficient antioxidant defenses mechanisms [23][41]. This stress activates inflammatory agents, leading to damage of lipids, proteins, and DNA. Early chronic kidney disease stages show high OS, which worsens with declining kidney function and is notably higher in end-stage patients on HD compared to those on peritoneal dialysis [24][42]. Hemodialysis intensifies OS because the procedure activates prooxidant processes and depletes antioxidant molecules like vitamins and trace elements. Various factors contribute to this stress, including the interactions with non-compatible membranes and dialysate, anticoagulation, and the use of central venous catheters for vascular access and malfunctioning arteriovenous graft and fistulae [25][43].
Oxidative stress observed in uremia contributes to the inflammatory state of dialysis patients. Systemic inflammation is a multifactorial phenomenon but is partly attributed to the accumulation of uremic toxins that are difficult to remove (e.g., p-cresol and indoxyl sulfate) and bioincompatibility with the extracorporeal circuit, leading to complement activation and transcription of several proinflammatory cytokines, such as TNF, IL-1b, and IL-6 [26][44]. High levels of these cytokines are associated with lower survival in dialysis [27][45] and studies indicate that inflammation correlates strongly with atherosclerosis, with IL-6 being a good predictor of the inflammatory burden in this group of patients [28][46].
While some preliminary studies suggest that OL-HDF would modulate the expression of several genes involved in the pathogenesis of atherosclerosis, such as VEGF, PDGF, and IL-6, in peripheral mononuclear cells [29][47], other have shown that HDF would induce post-transcriptional modifications, decreasing the expression of genes involved in vascular damage. Switching from HD to OL-HDF has been shown to significantly reduce systemic inflammation and decrease the expression of pro-atherogenic miR-223 in endothelial vesicles, enhancing angiogenesis and reducing vascular calcification. The downregulation of miRNA-223 restores IGF-1 receptor gene expression, which prevents endothelial dysfunction and vascular calcification.
HDF has been shown to have a positive impact on oxidative stress compared to standard HD [30][31][32][49,50,51]. This could be attributed to the use of ultrapure dialysis fluid, hemodynamic improvement, better response to anemia treatment, and increased removal of medium- and large-weight molecules such as inflammatory mediators [25][43].
Preclinical studies and some prospective studies with small groups of patients have shown that convective transport of OL-HDF reduces cytokine levels and inflammatory parameters [33][52], as well as reducing the expression of proinflammatory CD14+ and CD16+ dendritic-cell-derived monocytes in patients undergoing HDF [34][53]. Along the same lines, a decrease in the inflammatory state has been observed in dialysis patients who switch to HDF, being more noticeable in diabetic patients. These results were explained by the downregulation of dendritic cell maturation and better control of the sympathetic nervous system by improving renalase levels in the group of patients submitted to HDF [35][54].
Therefore, although clinical trials aimed at finding specific benefits in this area are lacking, today, it is considered that high-volume HDF is the “least inflammatory” renal support technique, and it is possible that the decrease in chronic inflammation observed in these patients explains the decrease in chronic inflammation observed in these patients, which may explain the better results in long-term survival [26][36][44,55].
