Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Atul Pandey.
Sleep is essential for the survival of most living beings. Numerous researchers have identified a series of genes that are thought to regulate “sleep-state” or the “deprived state”. As sleep has a significant effect on physiology, lack of total sleep, or particularly rapid eye movement (REM) sleep, for a prolonged period would have a profound impact on various body tissues. REM sleep deprivation affected a total of 652 genes in the brain and 426 genes in the liver. Only 23 genes were affected commonly, 10 oppositely, and 13 similarly across brain and liver tissue. Nine-day REM sleep deprivation differentially affects genes and processes in the brain and liver of rats.
- rapid eye movement sleep deprivation
- differential gene expression in brain and liver tissue
1. Introduction
Sleep is a universal phenomenon but still lack fundamental knowledge of its overall functions and purpose. However, most comparative sleep data exist for terrestrial vertebrates, with much less known about sleep in invertebrates [1]. Though, recently the scientific community has sought to characteristic sleep in non-mammalian species like the fruit fly (Drosophila melanogaster) [2[2][3][4],3,4], the zebrafish (Danio rerio) [5[5][6][7],6,7], the nematode (Caenorhabditis elegans) [8], and bees (Apis mellifera, and Bombus terrestris) [9,10,11,12][9][10][11][12]. Prolonged sleep deprivation is fatal in many of the animals studied, except for pigeons, and several studies have sought to address how sleep promotes survival in rodents and primates [13,14,15,16][13][14][15][16]. Despite the lack of general knowledge regarding the functions of sleep, loss of sleep has been shown to drastically alter the physiology of many of the animals studied thus far [17,18,19][17][18][19]. The degree of physiological changes and the fatality that sleep loss brings about often vary depending upon the nature and duration of sleep deprivation [20,21][20][21]. Many theories have been proposed to explain the evolutionary significance and functions of sleep, which includes “null” and “synaptic plasticity” theories [22,23][22][23]. Recent advancements in sleep research has shed light on two major functions of sleep-reducing synaptic potentiation and waste clearance mediated by glymphatic system [24,25,26][24][25][26]. Thus, sleep seems to have specific, overarching functions for all species that depend on it [19]. While a single characterization cannot be ascribed to sleep, numerous studies link its loss to detrimental effects on metabolism, behavior, immunity, cellular functions, and hormonal regulations across species [27,28,29,30][27][28][29][30]. There are some mechanisms that are associated with behavioral plasticity that are dependent on sociality or physiological state regarding sleep regulation [12,31][12][31]. Additionally, in Drosophila, not all stages of sleep are necessary for basic survival, but questions relating to the critical functions of sleep, plasticity, and its overall importance are still being explored [31].
Rapid eye movement (REM) sleep is an essential part of sleep and is present only in avians and mammals, with the exception of reptiles, in which REM sleep has only been recently discovered [32]. Unearthed thus far, the functional aspects of REM sleep includes mainly memory consolidation, brain maturation, muscle re-aeration, special memory acquisition, and maintenance of general physiological mechanisms of the body [33,34,35,36,37,38,39][33][34][35][36][37][38][39]. In the brain, REM sleep is involved in the reorganization of hippocampal excitability, pruning and maintenance of new synapses during development, and learning and memory consolidation [40,41,42][40][41][42]. Some recent studies also suggest that lack of REM sleep may cause cell death of somatic cells and neurons [43,44,45][43][44][45]. Outside of the brain, deprivation of the REM sleep was found to be associated with acute phase response in the liver, increased synthesis of pro-inflammatory cytokines such as IL1β, IL-6, and IL-12, and an increase in liver enzymes, alanine transaminase and aspartic transaminase [46]. In addition, REM sleep deprivation induces the production of reactive oxygen species (ROS) and causes inflammation [47] and an increase in nitric oxide (NO) in hepatocytes, along with an increase in sensitivity to oxidative stress by the hepatocytes [48]. REM loss also affected the weight and content of nucleic acid in the liver [49]. REM loss was also found to be further associated with oxidative stress and liver circadian clock gene expression [50]. An elevated increase in metabolic rate and UCP1 gene expression is reported in response to chronic REM sleep loss in the brown adipose tissue of rats [51]. Recently, REM sleep loss has been found to be associated with blood-brain barrier function regulation and metabolic changes [52,53][52][53].
2. Differential Gene Expression in Brain and Liver Tissue of Wistar Rats after Rapid Eye Movement Sleep Deprivation
Several of the aforementioned genes that were commonly associated with brain and liver tissue, and any combination of direction of change, were found in previous literature regarding sleep and REM. Several genes of the solute carrier (Slc) family (Table S4) were up- and down-regulated in the brain and liver, respectively, except for slc2a12, which was down-regulated in both the brain and liver. Previously, genes of the slc family were reported to be associated with glucose homeostasis, and slc17a8 is down-regulated in Tinaja cave fish in response to sleep deprivation [104][54]. Slc38a5a is up-regulated in response to sleep deprivation when glucose levels drop and circulating amino acid levels increase [105][55].
REM sleep deprivation is found to be associated with modification of expression of long-term potentiation in the visual cortex of immature rats [119][56], and the research report up-regulation of structural constituents of ribosomes, translation regulation activity, while dopamine receptor-signaling pathway, dopaminergic, cholinergic, GABAergic regulation of synaptic transmission, serotonin binding, and receptor activity were down-regulated in the brain. The dopamine receptor-signaling pathways regulating sleep, learning, and its plasticity are well known [83,84][57][58].
Processes and pathways in the liver following REM sleep deprivation are largely associated with metabolism and the immune system. Many metabolic processes and cellular metabolic processes such as gluconeogenesis, the triglyceride metabolic process, the negative regulation of fatty acid biosynthetic process, oxidation reduction, and the arachidonic acid metabolic process were up-regulated in the liver in response to REM loss. Whole body energy expenditure decreases by 15–35 percent, with the lowest expenditure during slow-wave sleep and a marginally higher expenditure during REM sleep [120][59], and sleep restriction involves reduced muscle glucose uptake, elevated blood glucose production, and pancreatic β-cell dysfunction [121,122][60][61]. An increasing body of evidence indicates that Obstructive Sleep Apnea Syndrome is associated with a variety of metabolic alterations such as dyslipidemia, insulin resistance, and glucose intolerance [123][62]. REM sleep impairs glucose metabolism, which is involved in intermittent hypoxemia [124][63]. An up-regulation of gluconeogenesis may serve as a mechanism to compensate for hypoxemia due to prolonged REM loss. The GO terms related to homeostatic processes, such as cholesterol homeostasis, nitric oxide homeostasis, fatty acid homeostasis, retina homeostasis, and cytosolic calcium ion homeostasis, are associated with genes that were up-regulated in the liver, while T cell homeostasis and other processes associated with the immune system were down-regulated. The immune functions of sleep and associated diseases have been studied [125[64][65],126], and it has been evidenced that the immune system is compromised by lack of sleep [127][66].
REM sleep loss negatively affects several genes linked to neuroactive ligand-receptor interaction pathways in the brain, primarily gamma-Aminobutyric acid, the Human Thrombin receptor, and associated receptor signaling dopamine. A recent review of sleep and protein-dependent synaptic plasticity indicated that sleep deprivation impairs many of the related biological and physiological processes [128][67]. Many of the pathways in the liver that have been up-regulated are linked to metabolism, immunity, and depression. On the other hand, only a few down-regulated pathways in the liver have been established, which include nitrogen metabolism and circadian rhythm. The findings further support the secondary hypothesis that REM sleep loss affects the processes and pathways related to synaptic potentiation and learning and memory (Figure 81A) and processes related to homeostasis and immunity in the liver (Figure 81A–C).
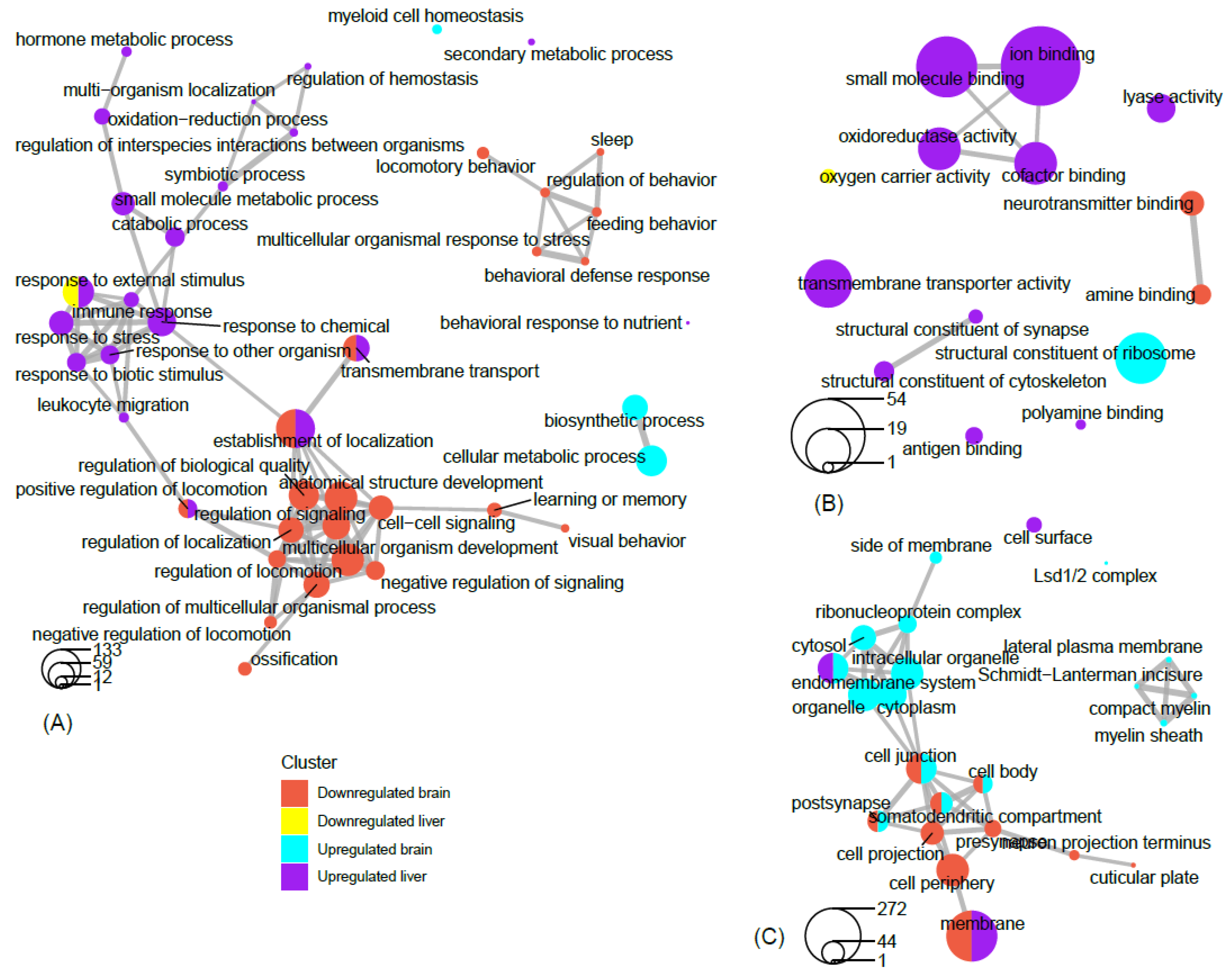 Network analysis of filtered GO terms allowed for the visualization of major themes and the connectivity of processes across brain and liver tissue in rats deprived of REM sleep (Figure 81). Several biological processes, such as positive regulation of locomotion, establishment of localization, and transmembrane transport were terms that were significantly enriched for genes that were both down-regulated in the brain and up-regulated in the liver. Interestingly, responses to external stimuli genes were found to be both positively and negatively affected in the liver, indicating the up- and down-regulation of separate sets of genes associated with this term (Figure 81A). There was no connectivity between terms in the molecular function category; however, terms associated with metabolism and transport, such as oxidoreductase activity, small molecule binding, iron binding, and cofactor binding, were each up-regulated in the liver (Figure 81B). Networking of terms in the cellular component category returned the GO terms cell junction, cell body, post synapse, and somatodendritic compartment, that were up- and down-regulated in the brain and liver, respectively (Figure 81C). To summarize a major theme, some processes that were mainly up-regulated in the liver were also down-regulated in the brain as a result of REM sleep loss. One possible explanation for this is that REM sleep loss influences processes linked to the fear response of the brain and locomotive activity related to the peripheral circadian clock, hemoglobin level, and transport of oxygen throughout the liver. The evidence suggests that the genes and processes involved are highly contrasted between the brain and the liver; however, some processes may be connected across major organs in response to REM sleep loss and should be investigated in the future.
Previous studies have shown that there are transcript level variations in many genes involved in the regulation of reactive oxygen species (ROS), including heme oxygenase, superoxide dismutase, and catalase, in patients with obstructive sleep apnea [129][68]. Similarly, REM sleep has recently been found to be associated with acute phase response and ROS stress in the liver [46,48][46][48]. REM sleep loss also affected several genes such as prostaglandin-endoperoxide synthase (Ptgs2), B-cell lymphoma 2 (Bcl-2), Proto-Oncogene, Tyrosine Kinase receptor (Kit), KRAS Proto-Oncogene (K-Ras), and Fos Proto-Oncogene (Fos), which are marked in cancer pathways. A number of recent studies have shown that sleep dysfunction/loss and cancer processes are closely related [130,131,132,133,134,135,136][69][70][71][72][73][74][75]. However, some emerging evidence also suggests that sleep loss/insomnia prior to the onset of cancer is independently associated with cancer risk [131,135,137,138][70][74][76][77]. Ptgs2, an enzyme, plays a key role in various pathological processes by catalyzing conversion of arachidonic acid to prostaglandins [139][78]. Studies have shown that overexpression of Ptgs2 is associated with angiogenesis, metastases, and immunosuppression [75,76][79][80]. Pgst2 is also found to be associated with the chemoresistance of some malignant tumors, including liver, pancreatic, lung, esophageal, and gastric cancers [77,78,79][81][82][83]. Inhibition of Ptgs2 effectively increased the sensitivity of tumors to drugs [140][84]. Similarly, Bcl-2, Kit, K-Ras, and Fos genes have been found to be associated with cancer [141,142,143,144][85][86][87][88]. These genes play an important role in the sleep-wake cycle regulation and are shown to be correlated with sleep [43,44,145,146,147][43][44][89][90][91]. At the same time, the glycerophospholipid metabolism pathway was found to be significantly up-regulated in the liver (Figure S4). These include the genes Phospholipase, PLa2g, Phosphatidylcholine 2-Acylhydrolase 12A Pla2g12a, Glycerol-3-Phosphate Dehydrogenase 2, Gpd2, CDP-Diacylglycerol Synthase 2, Cds2, and Phospholipid Phosphatase 2, Plpp2. The PLa2g associates with neurodegeneration and elevated mitochondrial lipid peroxidation and dysfunction [148,149,150][92][93][94]. The PLa2g is further found to be positively associated with sleep loss and psoriasis in humans [151,152][95][96]. Similarly, the Gpd2 gene is found to be associated with intellectual disability in humans [153][97] and positively affected due to circadian desynchrony in mice [154][98]. The chronic sleep deprivation in rats affected the protein profile of Gpd2 in hypothalamic astrocytes [103][99]. The functional aspect of the other genes affected (e.g., Pla2g12a, Cds2, and Plpp2) is lacking and needs further exploration. These findings further support the idea of REM sleep being related to restorative functions against diseases and oxidative stress.
Many KEGG pathways were associated with genes that were either significantly up- or down-regulated in the brain or liver as a result of REM sleep loss. The KEGG pathway map demonstrates that many of the genes for ribosomal proteins that are involved in protein synthesis processes were up-regulated in the brain by REM sleep loss. Indeed, research has shown that long-term sleep loss has been found to control several genes in the brain that are linked to the DNA binding/regulation of transcription, immunoglobulin synthesis, and stress response [55,90][100][101]. Contrary to the notion that Homer-1a is a key brain molecule in response to sleep loss in mice, no effect on gene expression of the Homer gene, which suggests that its regulation is modulated during other stages of sleep or is an organism-specific phenomenon [81][102]. The results underscore the complexity of sleep loss and its associated consequences, and sleep phase-, species-, and/or tissue-specific considerations rather than overarching, vague generalizations are required to deeply understand the phenomenon.
Additionally, REM sleep loss negatively affected several genes linked to neuroactive ligand-receptor interaction pathways in the brain, primarily related to gamma-Aminobutyric acid, Human Thrombin receptor, and associated receptor signaling dopamine. A recent review of sleep and protein-dependent synaptic plasticity indicated that sleep deprivation impairs many of the related biological and physiological processes [128][67]. Many of the pathways in the liver that have been up-regulated are linked to metabolism, immunity, and depression. On the other hand, only a few down-regulated pathways in the liver have been established, which include nitrogen metabolism and circadian rhythm.
Findings across studies are inconsistent regarding REM sleep deprivation and locomotor behavior and pain tolerance in rodents. Several studies have shown that REM sleep loss induces locomotor activity [92[103][104][105],155,156], while others have shown decreased locomotor activity [157][106]. The lack of a consistent explanation could be related to procedural changes in the methods of a given study, such as the degree of REM sleep loss. A widely accepted view in the scientific community is that sleep deprivation decreases pain tolerance and increases the transmission of pain in multiple chronic pain conditions [160,161,162,163,164,165][107][108][109][110][111][112]. Nonetheless, selective REM sleep deprivation is correlated with enhanced placebo analgesia effects [170][113]. Similarly, consistency exists between REM sleep loss and its association with the perception of pain [171][114]. Perhaps sleep in general and short-term REM sleep deprivation lower the pain threshold, while long-term sleep deprivation increases the pain threshold. REM sleep deprivation and pain is significantly correlated with environmental conditions (e.g., dry or wet conditions), with pain sensitivity enhanced in dry test conditions but no different in wet conditions. [172][115]. This suggests that further work is needed to understand deeply the relationship between the experience of pain and lack of sleep. Furthermore, a recent microarray analysis shows that Hspa5 gene expression increases not only in the brain but also in the liver as sleep deprivation increases [81][102]. Genes such as Wee1, Slc2a12, Hrk, and Fam110b were commonly down-regulated in both the brain and liver. Currently, however, there are disagreements about the relationship between expression of genes associated with locomotor behavior and pain tolerance, an area that is open for future research.
Network analysis of filtered GO terms allowed for the visualization of major themes and the connectivity of processes across brain and liver tissue in rats deprived of REM sleep (Figure 81). Several biological processes, such as positive regulation of locomotion, establishment of localization, and transmembrane transport were terms that were significantly enriched for genes that were both down-regulated in the brain and up-regulated in the liver. Interestingly, responses to external stimuli genes were found to be both positively and negatively affected in the liver, indicating the up- and down-regulation of separate sets of genes associated with this term (Figure 81A). There was no connectivity between terms in the molecular function category; however, terms associated with metabolism and transport, such as oxidoreductase activity, small molecule binding, iron binding, and cofactor binding, were each up-regulated in the liver (Figure 81B). Networking of terms in the cellular component category returned the GO terms cell junction, cell body, post synapse, and somatodendritic compartment, that were up- and down-regulated in the brain and liver, respectively (Figure 81C). To summarize a major theme, some processes that were mainly up-regulated in the liver were also down-regulated in the brain as a result of REM sleep loss. One possible explanation for this is that REM sleep loss influences processes linked to the fear response of the brain and locomotive activity related to the peripheral circadian clock, hemoglobin level, and transport of oxygen throughout the liver. The evidence suggests that the genes and processes involved are highly contrasted between the brain and the liver; however, some processes may be connected across major organs in response to REM sleep loss and should be investigated in the future.
Previous studies have shown that there are transcript level variations in many genes involved in the regulation of reactive oxygen species (ROS), including heme oxygenase, superoxide dismutase, and catalase, in patients with obstructive sleep apnea [129][68]. Similarly, REM sleep has recently been found to be associated with acute phase response and ROS stress in the liver [46,48][46][48]. REM sleep loss also affected several genes such as prostaglandin-endoperoxide synthase (Ptgs2), B-cell lymphoma 2 (Bcl-2), Proto-Oncogene, Tyrosine Kinase receptor (Kit), KRAS Proto-Oncogene (K-Ras), and Fos Proto-Oncogene (Fos), which are marked in cancer pathways. A number of recent studies have shown that sleep dysfunction/loss and cancer processes are closely related [130,131,132,133,134,135,136][69][70][71][72][73][74][75]. However, some emerging evidence also suggests that sleep loss/insomnia prior to the onset of cancer is independently associated with cancer risk [131,135,137,138][70][74][76][77]. Ptgs2, an enzyme, plays a key role in various pathological processes by catalyzing conversion of arachidonic acid to prostaglandins [139][78]. Studies have shown that overexpression of Ptgs2 is associated with angiogenesis, metastases, and immunosuppression [75,76][79][80]. Pgst2 is also found to be associated with the chemoresistance of some malignant tumors, including liver, pancreatic, lung, esophageal, and gastric cancers [77,78,79][81][82][83]. Inhibition of Ptgs2 effectively increased the sensitivity of tumors to drugs [140][84]. Similarly, Bcl-2, Kit, K-Ras, and Fos genes have been found to be associated with cancer [141,142,143,144][85][86][87][88]. These genes play an important role in the sleep-wake cycle regulation and are shown to be correlated with sleep [43,44,145,146,147][43][44][89][90][91]. At the same time, the glycerophospholipid metabolism pathway was found to be significantly up-regulated in the liver (Figure S4). These include the genes Phospholipase, PLa2g, Phosphatidylcholine 2-Acylhydrolase 12A Pla2g12a, Glycerol-3-Phosphate Dehydrogenase 2, Gpd2, CDP-Diacylglycerol Synthase 2, Cds2, and Phospholipid Phosphatase 2, Plpp2. The PLa2g associates with neurodegeneration and elevated mitochondrial lipid peroxidation and dysfunction [148,149,150][92][93][94]. The PLa2g is further found to be positively associated with sleep loss and psoriasis in humans [151,152][95][96]. Similarly, the Gpd2 gene is found to be associated with intellectual disability in humans [153][97] and positively affected due to circadian desynchrony in mice [154][98]. The chronic sleep deprivation in rats affected the protein profile of Gpd2 in hypothalamic astrocytes [103][99]. The functional aspect of the other genes affected (e.g., Pla2g12a, Cds2, and Plpp2) is lacking and needs further exploration. These findings further support the idea of REM sleep being related to restorative functions against diseases and oxidative stress.
Many KEGG pathways were associated with genes that were either significantly up- or down-regulated in the brain or liver as a result of REM sleep loss. The KEGG pathway map demonstrates that many of the genes for ribosomal proteins that are involved in protein synthesis processes were up-regulated in the brain by REM sleep loss. Indeed, research has shown that long-term sleep loss has been found to control several genes in the brain that are linked to the DNA binding/regulation of transcription, immunoglobulin synthesis, and stress response [55,90][100][101]. Contrary to the notion that Homer-1a is a key brain molecule in response to sleep loss in mice, no effect on gene expression of the Homer gene, which suggests that its regulation is modulated during other stages of sleep or is an organism-specific phenomenon [81][102]. The results underscore the complexity of sleep loss and its associated consequences, and sleep phase-, species-, and/or tissue-specific considerations rather than overarching, vague generalizations are required to deeply understand the phenomenon.
Additionally, REM sleep loss negatively affected several genes linked to neuroactive ligand-receptor interaction pathways in the brain, primarily related to gamma-Aminobutyric acid, Human Thrombin receptor, and associated receptor signaling dopamine. A recent review of sleep and protein-dependent synaptic plasticity indicated that sleep deprivation impairs many of the related biological and physiological processes [128][67]. Many of the pathways in the liver that have been up-regulated are linked to metabolism, immunity, and depression. On the other hand, only a few down-regulated pathways in the liver have been established, which include nitrogen metabolism and circadian rhythm.
Findings across studies are inconsistent regarding REM sleep deprivation and locomotor behavior and pain tolerance in rodents. Several studies have shown that REM sleep loss induces locomotor activity [92[103][104][105],155,156], while others have shown decreased locomotor activity [157][106]. The lack of a consistent explanation could be related to procedural changes in the methods of a given study, such as the degree of REM sleep loss. A widely accepted view in the scientific community is that sleep deprivation decreases pain tolerance and increases the transmission of pain in multiple chronic pain conditions [160,161,162,163,164,165][107][108][109][110][111][112]. Nonetheless, selective REM sleep deprivation is correlated with enhanced placebo analgesia effects [170][113]. Similarly, consistency exists between REM sleep loss and its association with the perception of pain [171][114]. Perhaps sleep in general and short-term REM sleep deprivation lower the pain threshold, while long-term sleep deprivation increases the pain threshold. REM sleep deprivation and pain is significantly correlated with environmental conditions (e.g., dry or wet conditions), with pain sensitivity enhanced in dry test conditions but no different in wet conditions. [172][115]. This suggests that further work is needed to understand deeply the relationship between the experience of pain and lack of sleep. Furthermore, a recent microarray analysis shows that Hspa5 gene expression increases not only in the brain but also in the liver as sleep deprivation increases [81][102]. Genes such as Wee1, Slc2a12, Hrk, and Fam110b were commonly down-regulated in both the brain and liver. Currently, however, there are disagreements about the relationship between expression of genes associated with locomotor behavior and pain tolerance, an area that is open for future research.

Figure 81. Network view of GO term association. Network plots of the top filtered GO terms, depicting the degree of connectivity within and between terms of enriched genes that are up-regulated in the brain, up-regulated in the liver, down-regulated in the brain, and down-regulated in the liver. The circles-legend at the bottom of each left-hand corner indicates the number of genes that are enriched for a given term. Connecting lines indicate a significant degree of semantic similarity between terms. Biological process (A), cellular component (B), and molecular function (C). GO terms were filtered (level = 3) to reduce redundancy and capture major categorical themes prior to visualization of connectivity in network plots, which were designed in R using the cluster Profiler package. Plots of filtered GO terms contained the top 20 significant categories, respectively, per subject cluster.
References
- Kelly, M.L.; Collin, S.P.; Hemmi, J.M.; Lesku, J.A. Evidence for Sleep in Sharks and Rays: Behavioural, Physiological, and Evolutionary Considerations. Brain Behav. Evol. 2020.
- Hendricks, J.C.; Finn, S.M.; Panckeri, K.A.; Chavkin, J.; Williams, J.A.; Sehgal, A.; Pack, A.I. Rest in Drosophila is a sleep-like state. Neuron 2000, 25, 129–138.
- Shaw, P.J.; Cirelli, C.; Greensoan, R.J.; Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 2000, 287, 1834–1837.
- Zimmerman, J.E.; Naidoo, N.; Raizen, D.M.; Pack, A.I. Conservation of sleep: Insights from non-mammalian model systems. Trends Neurosci. 2008, 31, 371–376.
- Yokogawa, T.; Marin, W.; Faraco, J.; Pézeron, G.; Appelbaum, L.; Zhang, J.; Rosa, F.; Mourrain, F.; Mignot, E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007, 5, 2379–2397.
- Prober, D.A.; Rihel, J.; Onah, A.A.; Sung, R.-J.; Schier, A.F. Hypocretin/Orexin Overexpression Induces An Insomnia-Like Phenotype in Zebrafish. J. Neurosci. 2006, 26, 13400–13410.
- Zhdanova, I.V.; Wang, S.Y.; Leclair, O.U.; Danilova, N.P. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001, 903, 263–268.
- Raizen, D.M.; Zimmerman, J.E.; Maycock, M.H.; Ta, U.D.; You, Y.J.; Sundaram, M.V.; Pack, A.I. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 2008, 451, 569–572.
- Eban-Rothschild, A.; Bloch, G. Circadian rhythms and sleep in honey bees. Honeybee Neurobiol. Behav. 2012, 31–45.
- Eban-Rothschild, A.D.; Bloch, G. Differences in the sleep architecture of forager and young honeybees (Apis mellifera). J. Exp. Biol. 2008, 211, 2408–2416.
- Klein, B.A.; Seeley, T.D. Work or sleep? Honeybee foragers opportunistically nap during the day when forage is not available. Anim. Behav. 2011, 82, 77–83.
- Nagari, M.; Gera, A.; Jonsson, S.; Bloch, G. Bumble Bee Workers Give Up Sleep to Care for Offspring that Are Not Their Own. Curr. Biol. 2019, 29, 3488–3493.
- Shaw, P.J.; Tortoni, G.; Greenspan, R.J.; Robinson, D.F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 2002.
- Stephenson, R.; Chu, K.M.; Lee, J. Prolonged deprivation of sleep-like rest raises metabolic rate in the Pacific beetle cockroach, Diploptera punctata (Eschscholtz). J. Exp. Biol. 2007.
- Montagna, P.; Lugaresi, E. Agrypnia Excitata: A generalized overactivity syndrome and a useful concept in the neurophysiopathology of sleep. Clin. Neurophysiol. 2002.
- Newman, S.M.; Paletz, E.M.; Rattenborg, N.C.; Obermeyer, W.H.; Benca, R.M. Sleep deprivation in the pigeon using the Disk-Over-Water method. Physiol. Behav. 2008.
- Rechtschaffen, A.; Gilliland, M.A.; Bergmann, B.M.; Winter, J.B. Physiological correlates of prolonged sleep deprivation in rats. Science 1983, 221, 182–184.
- Webb, W.B.; Agnew, H.W. Sleep deprivation, age, and exhaustion time in the rat. Science 1962, 136, 1122.
- Cirelli, C.; Tononi, G. Is sleep essential? PLoS Biol. 2008, 6, 1605–1611.
- Mirsky, A.F.; Cardon, P.V. A comparison of the behavioral and physiological changes accompanying sleep deprivation and chlorpromazine administration in man. Electroencephalogr. Clin. Neurophysiol. 1962.
- Kayser, M.S.; Mainwaring, B.; Yue, Z.; Sehgal, A. Sleep deprivation suppresses aggression in Drosophila. Elife 2015.
- Rial, R.V.; Nicolau, M.C.; Gamundí, A.; Akaârir, M.; Aparicio, S.; Garau, C.; Tejada, S.; Roca, C.; Gené, L.; Moranta, D.; et al. The trivial function of sleep. Sleep Med. Rev. 2007.
- Rattenborg, N.C.; Lesku, J.A.; Martinez-Gonzalez, D.; Lima, S.L. The non-trivial functions of sleep. Sleep Med. Rev. 2007.
- Tononi, G.; Cirelli, C. Sleep and synaptic down-selection. Eur. J. Neurosci. 2020.
- Tononi, G.; Cirelli, C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014.
- Mendelsohn, A.R.; Larrick, J.W. Sleep Facilitates Clearance of Metabolites from the Brain: Glymphatic Function in Aging and Neurodegenerative Diseases. Rejuvenation Res. 2013.
- Opp, M.R.; Baracchi, F. Sleep and immune function. Curr. Adv. Sleep Biol. 2009.
- Opp, M.R.; Krueger, J.M. Sleep and immunity: A growing field with clinical impact. Brain Behav. Immun. 2015.
- Mullington, J.M.; Haack, M.; Toth, M.; Serrador, J.M.; Meier-Ewert, H.K. Cardiovascular, Inflammatory, and Metabolic Consequences of Sleep Deprivation. Prog. Cardiovasc. Dis. 2009.
- Cirelli, C. Cellular consequences of sleep deprivation in the brain. Sleep Med. Rev. 2006.
- Geissmann, Q.; Beckwith, E.J.; Gilestro, G.F. Most sleep does not serve a vital function. Evidence from Drosophila melanogaster. BioRxiv 2018.
- Shein-Idelson, M.; Ondracek, J.M.; Liaw, H.P.; Reiter, S.; Laurent, G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 2016.
- Boyce, R.; Glasgow, S.D.; Williams, S.; Adamantidis, A. Sleep research: Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 2016, 352, 812–816.
- Graves, L.; Heller, E.; Pack, A.; Abel, T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 2003, 10, 168–176.
- Kumar, T.; Jha, S.K. Sleep Deprivation Impairs Consolidation of Cued Fear Memory in Rats. PLoS ONE 2012, 7.
- Youngblood, B.D.; Zhou, J.; Smagin, G.N.; Ryan, D.H.; Harris, R.B.S. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol. Behav. 1997, 61, 249–256.
- Chokroverty, S. Physiological changes of sleep. Sleep Disord. Med. Basic Sci. Tech. Consid. Clin. 2017, 153–194.
- Mallick, B.N.; Singh, S.; Pal, D. Role of alpha and beta adrenoceptors in locus coeruleus stimulation-induced reduction in rapid eye movement sleep in freely moving rats. Behav. Brain Res. 2005, 158, 9–21.
- Mônico-Neto, M.; Dáttilo, M.; Ribeiro, D.A.; Lee, K.S.; de Mello, M.T.; Tufik, S.; Antunes, H.K.M. REM sleep deprivation impairs muscle regeneration in rats. Growth Factors 2017.
- Van Der Helm, E.; Yao, J.; Dutt, S.; Rao, V.; Saletin, J.M.; Walker, M.P. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr. Biol. 2011, 21, 2029–2032.
- Grosmark, A.D.; Mizuseki, K.; Pastalkova, E.; Diba, K.; Buzsáki, G. REM Sleep Reorganizes Hippocampal Excitability. Neuron 2012, 75, 1001–1007.
- Li, W.; Ma, L.; Yang, G.; Gan, W.B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017, 20, 427–437.
- Somarajan, B.I.; Khanday, M.A.; Mallick, B.N. Rapid eye movement sleep deprivation induces neuronal apoptosis by noradrenaline acting on alpha1 adrenoceptor and by triggering mitochondrial intrinsic pathway. Front. Neurol. 2016, 7.
- Pandey, A.; Kumar, D.; Ray, G.; Kar, S. Rapid eye movement sleep deprivation causes apoptotic cell-death of the hepatocytes in rat. BioRxiv 2018.
- Biswas, S.; Mishra, P.; Mallick, B.N. Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience 2006, 142, 315–331.
- Pandey, A.K.; Kar, S.K. REM sleep deprivation of rats induces acute phase response in liver. Biochem. Biophys. Res. Commun. 2011, 410, 242–246.
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J. Interf. Cytokine Res. 2009.
- Pandey, A.; Kar, S.K. Rapid Eye Movement sleep deprivation of rat generates ROS in the hepatocytes and makes them more susceptible to oxidative stress. Sleep Sci. 2018.
- Balestrieri, S.; D’Onofrio, G.; Giuditta, A. Deprivation of paradoxical sleep effect on weight and nucleic acid content of liver and brain. Neurochem. Res. 1980.
- Li, T.; Cao, R.; Xia, R.; Xia, Z. Effects of 72 Hours Sleep Deprivation on Liver Circadian Clock Gene Expression and Oxidative Stress in Rats. Yangtze Med. 2017.
- Koban, M.; Swinson, K.L. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2005.
- Gómez-González, B.; Hurtado-Alvarado, G.; Esqueda-León, E.; Santana-Miranda, R.; Rojas-Zamorano, J.Á.; Velázquez-Moctezuma, J. REM sleep loss and recovery regulates blood-brain barrier function. Curr. Neurovasc. Res. 2013, 10, 197–207.
- Venancio, D.P.; Suchecki, D. Prolonged REM sleep restriction induces metabolic syndrome-related changes: Mediation by pro-inflammatory cytokines. Brain Behav. Immun. 2015.
- Obholzer, N.; Wolfson, S.; Trapani, J.G.; Mo, W.; Nechiporuk, A.; Busch-Nentwich, E.; Seiler, C.; Sidi, S.; Söllner, C.; Duncan, R.N.; et al. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J. Neurosci. 2008.
- McGaugh, S.E.; Passow, C.N.; Jaggard, J.B.; Stahl, B.A.; Keene, A.C. Unique transcriptional signatures of sleep loss across independently evolved cavefish populations. J. Exp. Zool. Part B Mol. Dev. Evol. 2020.
- Shaffery, J.P.; Sinton, C.M.; Bissette, G.; Roffwarg, H.P.; Marks, G.A. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 2002.
- Volkow, N.D.; Tomasi, D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Logan, J.; Benveniste, H.; Kim, R.; Thanos, P.K.; Ferré, S. Evidence that sleep deprivation down regulates dopamine D2R in ventral striatum in the human brain. J. Neurosci. 2012.
- França, A.S.C.; Lobão-Soares, B.; Muratori, L.; Nascimento, G.; Winne, J.; Pereira, C.M.; Jeronimo, S.M.B.; Ribeiro, S. D2 dopamine receptor regulation of learning, sleep and plasticity. Eur. Neuropsychopharmacol. 2015.
- Katayose, Y.; Tasaki, M.; Ogata, H.; Nakata, Y.; Tokuyama, K.; Satoh, M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism 2009.
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439.
- Donga, E.; Van Dijk, M.; Van Dijk, J.G.; Biermasz, N.R.; Lammers, G.J.; Van Kralingen, K.W.; Corssmit, E.P.M.; Romijn, J. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J. Clin. Endocrinol. Metab. 2010.
- Pamidi, S.; Aronsohn, R.S.; Tasali, E. Obstructive sleep apnea: Role in the risk and severity of diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2010.
- Iiyori, N.; Alonso, L.C.; Li, J.; Sanders, M.H.; Garcia-Ocana, A.; O’Doherty, R.M.; Polotsky, V.Y.; O’Donnell, C.P. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am. J. Respir. Crit. Care Med. 2007.
- Asif, N.; Iqbal, R.; Nazir, C.F. Human immune system during sleep. Am. J. Clin. Exp. Immunol. 2017, 6, 92.
- Besedovsky, L.; Lange, T.; Haack, M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019.
- Ruiz, F.S.; Andersen, M.L.; Martins, R.C.S.; Zager, A.; Lopes, J.D.; Tufik, S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immun. 2012.
- Grønli, J.; Soulé, J.; Bramham, C.R. Sleep and protein synthesis-dependent synaptic plasticity: Impacts of sleep loss and stress. Front. Behav. Neurosci. 2014.
- Hoffmann, M.S.; Singh, P.; Wolk, R.; Romero-Corral, A.; Raghavakaimal, S.; Somers, V.K. Microarray studies of genomic oxidative stress and cell cycle responses in obstructive sleep apnea. Antioxid. Redox Signal. 2007.
- Fiorentino, L.; Ancoli-Israel, S. Sleep dysfunction in patients with cancer. Curr. Treat. Options Neurol. 2007.
- Fang, H.F.; Miao, N.F.; Chen, C.D.; Sithole, T.; Chung, M.H. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: A nationwide nested case-control study. J. Cancer 2015.
- Sateia, M.J.; Lang, B.J. Sleep and cancer: Recent developments. Curr. Oncol. Rep. 2008.
- Shi, T.; Min, M.; Sun, C.; Zhang, Y.; Liang, M.; Sun, Y. Does insomnia predict a high risk of cancer? A systematic review and meta-analysis of cohort studies. J. Sleep Res. 2020.
- Martínez-Garćia, M.Á.; Martorell-Calatayud, A.; Nagore, E.; Valero, I.; Selma, M.J.; Chiner, E.; Landete, P.; Montserrat, J.M.; Carrera, C.; Peŕez-Gil, A.; et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur. Respir. J. 2014.
- Lin, C.L.; Liu, T.C.; Wang, Y.N.; Chung, C.H.; Chien, W.C. The association between sleep disorders and the risk of colorectal cancer in patients: A Population-based Nested Case–Control Study. In Vivo 2019.
- Sillah, A.; Watson, N.F.; Schwartz, S.M.; Gozal, D.; Phipps, A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018.
- Chiu, H.Y.; Huang, C.J.; Fan, Y.C.; Tsai, P.S. Insomnia but Not Hypnotics Use Associates with the Risk of Breast Cancer: A Population-Based Matched Cohort Study. J. Womens Health 2018.
- Sturgeon, S.R.; Luisi, N.; Balasubramanian, R.; Reeves, K.W. Sleep duration and endometrial cancer risk. Cancer Causes Control 2012.
- Liu, R.; Xu, K.P.; Tan, G.S. Cyclooxygenase-2 inhibitors in lung cancer treatment: Bench to bed. Eur. J. Pharmacol. 2015.
- Mignot, E. Why we sleep: The temporal organization of recovery. PLoS Biol. 2008, 6, 661–669.
- Cirelli, C.; Bushey, D. Sleep and wakefulness in Drosophila melanogaster. Ann. N. Y. Acad. Sci. 2008, 1129, 323–329.
- Cirelli, C.; LaVaute, T.M.; Tononi, G. Sleep and wakefulness modulate gene expression in Drosophila. J. Neurochem. 2005, 94, 1411–1419.
- Cirelli, C. Locus Ceruleus Control of State-Dependent Gene Expression. J. Neurosci. 2004, 24, 5410–5419.
- Jones, S.; Pfister-Genskow, M.; Benca, R.M.; Cirelli, C. Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J. Neurochem. 2008, 105, 46–62.
- Lin, X.M.; Luo, W.; Wang, H.; Li, R.Z.; Huang, Y.S.; Chen, L.K.; Wu, X.P. The role of prostaglandin-endoperoxide synthase-2 in chemoresistance of non-small cell lung cancer. Front. Pharmacol. 2019.
- Mueller, S.; Engleitner, T.; Maresch, R.; Zukowska, M.; Lange, S.; Kaltenbacher, T.; Konukiewitz, B.; Öllinger, R.; Zwiebel, M.; Strong, A.; et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature 2018.
- LoRusso, P.M.; Herbst, R.S.; Rischin, D.; Ranson, M.; Calvert, H.; Raymond, E.; Kieback, D.; Kaye, S.; Gianni, L.; Harris, A.; et al. Improvements in quality of life and disease-related symptoms in phase I trials of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 in non-small cell lung cancer and other solid tumors. Clin. Cancer. Res. 2003, 9, 2040–2048.
- Thomadaki, H.; Scorilas, A. BCL2 family of apoptosis-related genes: Functions and clinical implications in cancer. Crit. Rev. Clin. Lab. Sci. 2006.
- Cruse, G.; Metcalfe, D.D.; Olivera, A. Functional deregulation of KIT: Link to mast cell proliferative diseases and other neoplasms. Immunol. Allergy Clin. N. Am. 2014.
- Redline, S.; Tishler, P.V. The genetics of sleep apnea. Sleep Med. Rev. 2000.
- Cirelli, C.; Tononi, G. On the Functional Significance of c-fos Induction During the Sleep-waking Cycle. Sleep 2000.
- Cirelli, C.; Pompeiano, M.; Tononi, G. Sleep deprivation and c-fos expression in the rat brain. J. Sleep Res. 1995.
- Gregory, A.; Kurian, M.A.; Maher, E.R.; Hogarth, P.; Hayflick, S.J. PLA2G6-Associated Neurodegeneration. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; GeneReviews® ; University of Washington: Seattle, WA, USA, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 23 October 2020).
- Gregory, A.; Westaway, S.K.; Holm, I.E.; Kotzbauer, P.T.; Hogarth, P.; Sonek, S.; Coryell, J.C.; Nguyen, T.M.; Nardocci, N.; Zorzi, G.; et al. Neurodegeneration associated with genetic defects in phospholipase A2. Neurology 2008.
- Kinghorn, K.J.; Castillo-Quan, J.I.; Bartolome, F.; Angelova, P.R.; Li, L.; Pope, S.; Cochemé, H.M.; Khan, S.; Asghari, S.; Bhatia, K.P.; et al. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain 2015.
- Ollila, H. Genetics of Sleep, Sleep and Comorbidities: Connection at the Genetic Level; National Institute for Health and Welfare: Helsinki, Finland, 2013.
- Gao, Y.; Yi, X.; Ding, Y. Combined transcriptomic analysis revealed AKR1B10 played an important role in psoriasis through the dysregulated lipid pathway and overproliferation of keratinocyte. Biomed. Res. Int. 2017.
- Barge-Schaapveld, D.Q.C.M.; Ofman, R.; Knegt, A.C.; Alders, M.; Höhne, W.; Kemp, S.; Hennekam, R.C.M. Intellectual Disability and Hemizygous GPD2 Mutation. Am. J. Med. Genet. Part A 2013.
- Barclay, J.L.; Husse, J.; Bode, B.; Naujokat, N.; Meyer-Kovac, J.; Schmid, S.M.; Lehnert, H.; Oster, H. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE 2012.
- Kim, J.H.; Kim, J.H.; Cho, Y.E.; Baek, M.C.; Jung, J.Y.; Lee, M.G.; Jang, I.S.; Lee, H.O.; Suk, K. Chronic sleep deprivation-induced proteome changes in astrocytes of the rat hypothalamus. J. Proteome Res. 2014.
- Cirelli, C.; Tononi, G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000.
- Cirelli, C.; Faraguna, U.; Tononi, G. Changes in brain gene expression after long-term sleep deprivation. J. Neurochem. 2006.
- Maret, S.; Dorsaz, S.; Gurcel, L.; Pradervand, S.; Petit, B.; Pfister, C.; Hagenbuchle, O.; O’Hara, B.F.; Franken, P.; Tafti, M. Homer1a is a core brain molecular correlate of sleep loss. Proc. Natl. Acad. Sci. USA 2007, 104, 20090–20095.
- van Hulzen, Z.J.M.; Coenen, A.M.L. Paradoxical sleep deprivation and locomotor activity in rats. Physiol. Behav. 1981, 27, 741–744.
- Arriaga, F.; Dugovic, C.; Wauquier, A. Effects of lithium on dopamine behavioural supersensitivity induced by rapid eye movement sleep deprivation. Neuropsychobiology 1988.
- Albert, I.; Cicala, G.A.; Siegel, J. The behavioral effects of REM sleep deprivation in rats. Psychophysiology 1970.
- Asakura, W.; Matsumoto, K.; Ohta, H.; Watanabe, H. REM sleep deprivation decreases apomorphine-induced stimulation of locomotor activity but not stereotyped behavior in mice. Gen. Pharmacol. 1992.
- Drewes, A.M.; Svendsen, L.; Taagholt, S.J.; Bjerregård, K.; Nielsen, K.D.; Hansen, B. Sleep in rheumatoid arthritis: A comparison with healthy subjects and studies of sleep/wake interactions. Br. J. Rheumatol. 1998.
- Aǧargün, M.Y.; Tekeoǧlu, I.; Güneş, A.; Adak, B.; Kara, H.; Ercan, M. Sleep quality and pain threshold in patients with fibromyalgia. Compr. Psychiatry 1999.
- Raymond, I.; Nielsen, T.A.; Lavigne, G.; Manzini, C.; Choinière, M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain 2001.
- Schrimpf, M.; Liegl, G.; Boeckle, M.; Leitner, A.; Geisler, P.; Pieh, C. The effect of sleep deprivation on pain perception in healthy subjects: A meta-analysis. Sleep Med. 2015.
- Stroemel-Scheder, C.; Kundermann, B.; Lautenbacher, S. The effects of recovery sleep on pain perception: A systematic review. Neurosci. Biobehav. Rev. 2020.
- Roehrs, T.; Hyde, M.; Blaisdell, B.; Greenwald, M.; Roth, T. Sleep loss and REM sleep loss are hyperalgesic. Sleep 2006.
- Chouchou, F.; Chauny, J.M.; Rainville, P.; Lavigne, G.J. Selective REM sleep deprivation improves expectation-related placebo analgesia. PLoS ONE 2015.
- Smith, M.T.; Edwards, R.R.; Stonerock, G.L.; McCann, U.D. Individual variation in rapid eye movement sleep is associated with pain perception in healthy women: Preliminary data. Sleep 2005.
- Onen, S.H.; Alloui, A.; Jourdan, D.; Eschalier, A.; Dubray, C. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res. 2001.
More
