Mesenchymal stromal/stem cells (MSCs) are multipotent cells that can differentiate to various specialized cells, which have the potential capacity to differentiate properly and accelerate recovery in damaged sites of the body. This stem cell technology has become the fundamental element in regenerative medicine. As reactive oxygen species (ROS) have been reported to adversely influence stem cell properties, it is imperative to attenuate the extent of ROS to the promising protective approach with MSCs’ regenerative therapy. Oxidative stress also affects the culture expansion and longevity of MSCs. Therefore, there is great need to identify a method to prevent oxidative stress and replicative senescence in MSCs. Phosphatase and tensin homologue deleted on chromosome 10/Protein kinase B, PKB (PTEN/AKT) and the tumor suppressor p53 pathway have been proven to play a pivotal role in regulating cell apoptosis by regulating the oxidative stress and/or ROS quenching.
1. Introduction
Mesenchymal stromal/stem cells (MSCs) are multipotent stem cells that are present in almost all fetal and adult issues, which are characterized by their ability to differentiate into several specialized cells
[1]. MSCs are progenitors of the various important cells that have emerged as vital tools for medical engineering. MSCs have been isolated from a number of different tissues such as cord blood, placenta, and bone marrow, which are being investigated for several tissue repair, immune modulation, and so on
[2]. For example, studies have reported the transplantation of MSCs from bone marrow as a strategy for cardiac repair following myocardial infarction
[3]. Enhancing the viability of implanted MSCs and restoring cellular mechanisms are therefore serious in order to gain adequate outcomes with the therapy. Reactive oxygen species (ROS) and nonspecific inflammation have been hypothesized to lead to the loss of the transplanted MSCs from concerned sites
[4].
2. ROS Is Involved in the Differentiation and Senescence of MSCs
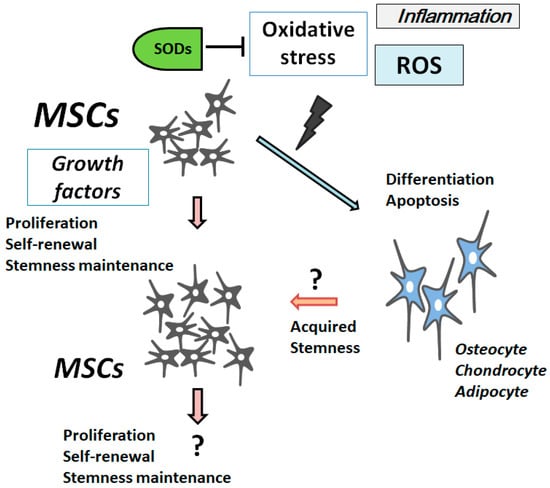
Generally, ROS inhibit MSCs’ proliferation, increase the senescence, and enhance several cell differentiations (
Figure 1). The ROS represent a group of oxygen-containing minor molecules, which react freely with various chemical structures. Examples of ROS include peroxides, superoxides, hydroxyl radicals, and singlet oxygen. ROS have been considered to bring cellular dysfunction and cell death/apoptosis via the damaging oxidation of cellular components. Now, it has been revealed ROS have important roles in normal cell physiology in addition to the pathology of several diseases such as tissue degenerative disorder and cancer
[5][8]. Whereas unregulated levels of ROS may be harmful, under physiological conditions, a regulated basal level of
Figure 1. Illustration of mesenchymal stromal/stem cells (MSCs) proliferation and/or differentiation in response to the extracellular growth factor stimulation and/or oxidative stress. The model shows that several triggers including oxidative stress, superoxide dismutases (SODs), reactive oxygen species (ROS), inflammation, and growth factors could affect MSCs and their destinations. SODs reduce some of the oxidative stress by dismuting superoxide. Note that some critical routes have been omitted for clarity.
3. Characterization of Superoxide Dismutases
A group of metal-containing enzymes named superoxide dismutases (SODs) have vigorous antioxidant roles categorized by their scavenging of ROS
[6][27]. The SODs have been thought to be the first line of protection arrangement against oxidative stresses, which implies that SODs can play an important defensive role in several cell apoptosis. Before some of ROS can oxidize critical DNA and/or proteins, SODs catalyze the reaction of superoxides to the less damaging/reactive hydrogen peroxide
[7][28]. The presence of metals such as Cu, Zn, Mn, or Fe may be essential for this function in the system
[8][29]. So, altered metal homeostasis in cells may be the cause of endogenous oxidative stress
[9][30]. Three types of SODs are known in mammalian species. The most abundant cytosolic enzyme is SOD1. The loss of SOD1 increases the total level of ROS, which is thought to trigger oxidative DNA damage to cells. It has been shown SOD1-null animals develop some age-related diseases
[10][31]. Amazingly, SOD1 may upturn the therapeutic potential of MSCs
[11][32]. SOD2 is located in the mitochondrial matrix
[12][33], which is the critical site of free radical production from the electron transportation chain. SOD2 is required for maintaining mitochondrial functions and reliability
[13][34]. One of the primary functions of SOD2 might be to protect mitochondrial DNA against oxidative damage
[14][35]. The SOD2 gene is subjected to regulation by a number of inflammatory cytokines and growth factors
[15][36]. It has been shown that SOD2 overexpression causes an increase in ATP production through energetic mitochondrial respiration
[16][37]. The induction of osteogenesis in MSCs is associated with an upregulation of SOD2 and a decrease in ROS levels
[17][38]. Additionally, the reduction of SOD2 diminishes the expression of an adipogenesis marker, which results in higher ROS production
[18][39]. SOD3 is secreted to the extracellular matrix in cells and tissues
[19][40]. Downregulation of SOD3 has been shown to lead DNA copy number change and/or hypermethylation in the promoter region of genes
[20][41]. It has been revealed that overexpressed SOD3 causes hypoxic accumulation of hypoxia inducible factor-1α (HIF1α) in cells
[21][42]. There is an increase in SOD3 expression with the differentiation of MSCs into adipocytes
[22][43]. In addition, SOD3 levels have been shown to decrease upon chondrogenesis
[22][43].
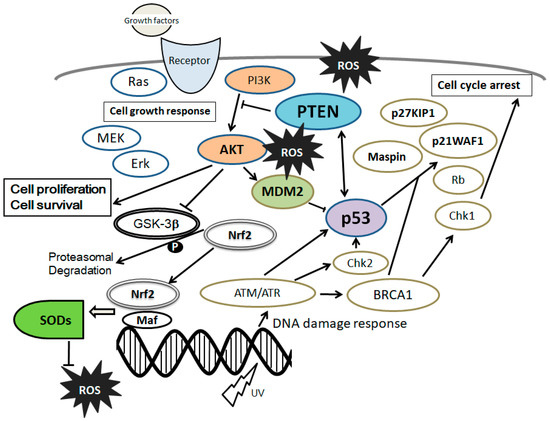
4. PI3K/AKT/PTEN and p53 Signaling Are Involved in Maintenance of MSCs’ Proliferation and Stemness
In MSCs, excess ROS can impair self-renewal, differentiation capacity, and proliferation
[23][44]. Concordantly, antioxidants stimulate MSCs’ proliferation
[24][45]. The MSCs’ stemness is maintained by inhibiting cellular senescence through a PI3K/AKT pathway
[25][46]. Moreover, the PI3K/AKT pathway has been shown to be involved in maintaining embryonic stem cell pluripotency
[26][47]. Activation of the PI3K/AKT signaling may have dynamic roles in maintaining the pluripotency of stem cells
[27][48], which is also involved in enhanced cell proliferation
[28][49] (
Figure 2). In addition, it has been reported that PI3K/AKT is associated with the regulation of stem cell fate
[29][50]. Stromal cell-derived factor 1 (SDF1) is an important chemokine in stem cell mobilization, and plays a critical role in the biological functions of MSCs by enhancing PI3K expression
[30][51]. Muc1 is a member of the carbohydrate-binding protein family that contributes to MSCs’ survival and stemness via the PI3K/AKT signaling
[31][52].
Figure 2. Schematic representation of the integrative model of tumor suppressor molecules signaling, including phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and p53 in response to the extracellular growth factor stimulation and/or oxidative stress. Typical examples of molecules known to act on the DNA damage response and cell proliferation or cell cycle progression via the regulatory intracellular pathway are shown. Note that some critical signaling has been omitted for clarity.
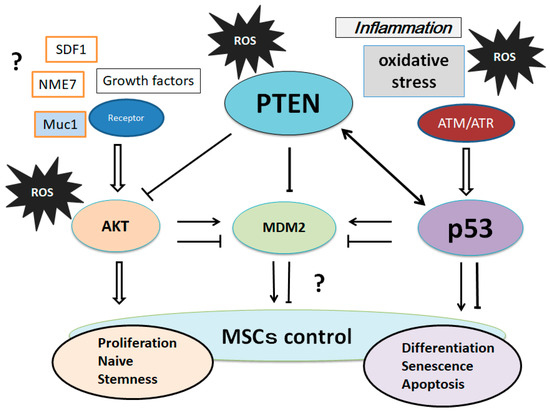
5. Involvement of PTEN-p53-AKT-MDM2 Loop in MSCs Regulation
It has been proposed that low levels of p53 induce cell cycle arrest, whereas high levels of p53 induce apoptosis
[32][69]. The PI3K/AKT activation runs into the inhibition of p53 by activating another tumor suppressor, MDM2
[33][70]. MDM2 is an oncoprotein that regulates tumorigenesis, whose mRNA level is regulated by p53 in response to oxidative stress and/or DNA damage
[34][71]. Subcellular localization of the MDM2 is post-translationally modulated by PI3K/AKT
[35][72]. Consequently, PI3K/AKT and p53 affect the process of apoptosis in opposed ways. In addition, there are cross-talks between AKT and p53 involving transcription as well as post-translational modifications
[35][36][72,73]. Moreover, the subsequent p53-induced expression of PTEN causes the p53–PTEN interaction, which suppresses the cell survival through PI3K/AKT signaling
[33][70]. PTEN associates with p53 and regulates the transcriptional activity of p53 by modulating its DNA binding
[37][74]. AKT kinase phosphorylates MDM2 to translocate into the nucleus, as mentioned formerly. In addition, PTEN is required for the maintenance of p53 acetylation, which is required for target gene transcription
[38][75]. PTEN has also been shown to interact with p53 and prevent its degradation. The p53 and MDM2 complex transports from the nucleus into the cytoplasm, where MDM2 serves as an E3 ubiquitin ligase
[39][76]. Attenuation of the PI3K/AKT pathway by PTEN protects p53 from MDM2-mediated degradation and inactivation. The levels of p53 could be positively related to the amount of oxidative DNA damage.
On the other hand, AKT activation can overcome both the p53-independent cell cycle checkpoint and apoptosis that is induced by the oxidative DNA damage. The PTEN-p53-MDM2-AKT loop in MSCs’ regulation now may become dominant (
Figure 3).
Figure 3. Suggestion of various molecular regulatory loops involving the PTEN-p53-AKT-MDM2 network on the controls of MSCs is shown. Interactions are shown as arrows to mean activation, while hammerheads mean inhibition. Expression of these molecules is regulated by genetic, epigenetic, and transcriptional changes, which may result in the MSCs’ regulation. Note that some critical pathways have been omitted for clarity.
It has been shown that zinc deficiency modulates the PTEN-p53-MDM2-AKT signaling axis
[40][80]. (
Figure 3) In addition, the enhanced proliferation potential of MSCs has been accompanied by the upregulation of multiple genes, including AKT and Mdm2
[41][81]. Many dietary compounds are known to have health benefits owing to their antioxidative and anti-inflammatory properties. Among them, the polyphenols from blueberries have been involved in the ultraviolet-activated p53/MDM2 DNA repair system by restoring the cell membrane potential
[42][82]. In addition, curcumin downregulates MDM2 and upregulates p53
[43][83], which exhibits cell protective effects. Chitosan is a deacetylated polysaccharide derivative of chitin that is contained in the shells of crustacean such as crabs and shrimps in nature, which activates the PI3K/AKT pathway
[44][84], and has also been shown to enhance mineral deposition during the osteogenic differentiation of MSCs
[45][85]. At these points, again, the PTEN-p53-MDM2-AKT loop becomes dominant in MSCs’ biology (
Figure 3). The microenvironment of cells/tissues could be driven by these diet ingredients.