Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yusof Kamisah and Version 2 by Lindsay Dong.
Cardiac diseases, such as myocardial infarction and heart failure, have become a major clinical problem globally. The accumulating data demonstrate that bioactive compounds with antioxidant and anti-inflammatory properties have favorable effects on clinical problems. Kaempferol is a flavonoid found in various plants; it has demonstrated cardioprotective properties in numerous cardiac injury models.
- cardiac disease
- flavonoid
- cardiac function
- antioxidant
- anti-inflammatory
- antifibrosis
- antiapoptosis
1. Introduction
Plants have a crucial role in human life and well-being. Humans use plants for food, clothing, furniture, and many other things. Plants have been used for medicinal purposes since ancient times. They produce secondary metabolites, such as flavonoids and terpenoids, for their self-defense [1][14]. Flavonoids are found abundantly in vegetables and fruits. The compounds are responsible for the pigmentation of yellow and red, as well as other colors in plants. They are divided into seven subclasses: flavonols (e.g., kaempferol and quercetin), flavones (e.g., luteolin and apigenin), flavanols (e.g., catechin and epicatechin), isoflavones (e.g., daidzein and genistein), anthocyanidins (e.g., cyanidin and delphinidin), flavonones (e.g., hesperetin and hesperidin), and chalcones (e.g., butein and naringenin chalcone) [2][3][4][15,16,17].
Flavonoids have been isolated from plants for various therapeutic effects. A study demonstrated that rutin and quercetin reduced Ang-II-induced cardiomyocyte hypertrophy by modulating mitogen-activated protein kinase (MAPK) [5][18]. On the other hand, kaempferol [6][11] and luteolin [7][19] protected against diabetic cardiomyopathy by regulating Kelch-like ECH-associated protein (Keap) and nuclear factor kappa B (NF-κB) signaling pathways. Hesperidin also diminishes injury following myocardial infarction in mice by modulating inflammatory response [8][20]. Therefore, plants have become a promising source of new drugs over the last four decades [9][21].
Kaempferol (3,4′,5,7-tetrahydroxyflavone) (Figure 1), a yellow crystalline compound, is a flavonol which is rich in various plants such as tea, broccoli, tomatoes [10][22], and beans (e.g., bitter bean) [11][23]. Many studies have demonstrated the pharmacological activities of this compound in various pathological conditions, such as cardiovascular disease [12][13][6,13] and cancer [14][24]. It protects against cardiac disease via antiapoptotic, antioxidative, anti-inflammatory, calcium regulatory, and antifibrotic mechanisms, as well as maintaining mitochondrial function, resulting in the amelioration of cardiac structure and function [15][16][17][25,26,27].
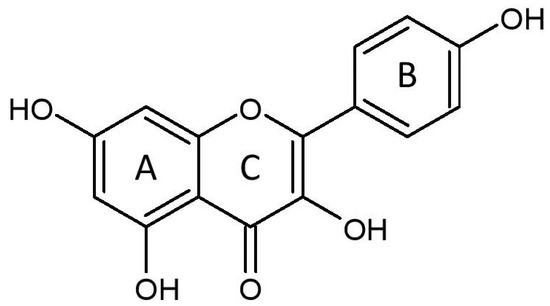
Figure 1.
The molecular structure of kaempferol, consisting of benzene rings A and B, as well as a heterocyclic ring C.
2. Effects on Cardiac Injury and Structure
Numerous models have been employed to determine the potential effects of kaempferol on cardiac injury. Various agents have been used in experimental animals and cardiomyocytes in vitro to induce cardiac injury, namely Ang II [12][6], isoprenaline [15][25], doxorubicin [18][7], cisplatin [19][8], 5-fluorouracil [20][9], phenylephrine [21][3], and clozapine [22][10], in addition to I/R [23][28] and aortic banding [21][3]. The inducers promote cardiac injury by elevating oxidative stress and inflammation, the culprits in cardiac injury. A burst of reactive oxygen species (ROS) production occurs upon the reintroduction of oxygen (reperfusion). Kaempferol reduces cardiac injury in animal models, as observed by the reduction in the release of cardiac injury markers, including creatine kinase, creatine kinase MB, troponin, and lactate dehydrogenase. It also curbs myocardial infarct size in rat hearts that undergo I/R [17][27] and in rats exposed to isoprenaline [15][25]. Meanwhile, in rats receiving 5-fluorouracil, kaempferol post-treatment reduced myocardial inflammatory changes, namely hyaline formation, necrosis, and hyperemia [20][9].
Kaempferol decreases levels of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) [6][19][21][3,8,11]. Both peptides are released in response to volumetric stretch of the atrial and ventricular walls [24][34]. Structurally, it also diminishes myocardial fiber derangement induced by cisplatin [19][8]. It reduces the detrimental effects of heart inducers by reducing interventricular septal thickness at diastole (IVSD), left ventricular internal diameter (IVIDd), and posterior wall (LVPWd) in diastole and systole [21][25][3,30], indicating the ability of kaempferol to decrease left ventricular wall thickening, a common phenomenon in cardiac remodeling. A change in left ventricular geometry triggers its remodeling [26][35]. The findings propose that kaempferol possesses antihypertrophic activity in the myocardium, confirmed by a reduction in cardiomyocyte size and heart weight-to-body weight ratio [12][19][21][25][3,6,8,30]. The protective effects are primarily via its blockade of ROS synthesis [21][3], which prevents subsequent events. Many events are involved in the development of cardiac hypertrophy and remodeling including fibrosis, apoptosis, and altered mitochondrial function, and the effects of kaempferol on the events will be discussed later.
Excessive activity of renin-angiotensin system could predispose to the development of left ventricular hypertrophy [27][36]. However, studies investigating the effects of kaempferol on the aspect are still lacking. Ang II, a proinflammatory peptide, is a principal substance in the renin–angiotensin system that plays a principal role in the pathogenesis of hypertrophy [28][37]. Even though many studies have demonstrated the positive effects of kaempferol on Ang-II-induced hypertrophy, the effects of the bioactive compound on the expression of Ang II; the angiotensin-converting enzyme?the Ang II synthesis enzyme; and its receptor, Ang II type 1 receptor, have not been explored. Understanding the mechanisms could shed more light on its potential pharmacological activities.
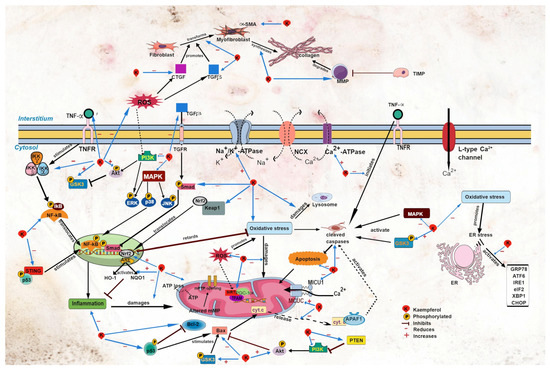
3. Effects on Cardiac Function
Kaempferol exhibits positive effects on cardiac function in various cardiac injury models. It improves left ventricular fractional shortening (LVFS) and ejection fraction (LVEF) [12][19][21][29][3,6,8,33]. Both parameters are used to detect left ventricular systolic function and are reduced in hearts with left ventricular failure [30][31][40,41]. Therefore, these parameters are used in the diagnosis of heart failure [32][42]. Other than the parameters, improvement of the systolic function by kaempferol (15 mM and 20 mg/kg/day) pretreatment are also evidenced by an increase in the maximal rate of rise (+dp/dtmax) and fall (−dp/dtmax) of left ventricular pressure in I/R- and isoprenaline-induced myocardial injury in rats [17][23][33][34][27,28,31,43], as well as left ventricular systolic pressure and developed pressure [17][35][36][2,27,32] in models of acute myocardial infarction and I/R injury. However, similar positive findings were not observed in left anterior descending coronary artery (LADCA)-ligation-induced heart failure in mice receiving 12 mg/kg/day for 3 days [37][44], possibly due to the shorter duration of kaempferol treatment compared with other studies. The beneficial effects of the flavonoid were also observed in diastolic function. It reduces left ventricular end-diastolic pressure (LVEDP) [25][33][34][35][36][2,30,31,32,43]. LVEDP measures left ventricular preload and diastolic compliance [38][45], indicating that kaempferol reduces preload, which is useful in angina. The reduction in the ratio of transmitral flow velocity/mitral annular velocity and the increase in left ventricular volume of diastole by kaempferol treatment indicate an improvement in myocardial diastolic function [12][21][3,6]. The betterment in diastolic function by kaempferol eventually indirectly improves the systolic function. Kaempferol possibly protects by preventing the loss of contractile function due to reducing the number of viable cardiomyocytes following an insult to myocardium, thereby hindering the development of cardiac remodeling. Consequently, myocardial inotropic and lusitropic properties are preserved by kaempferol. The restoration of both properties is crucial in maintaining a normal cardiac performance. Cardiac function is partly determined by myocardial cellular and molecular structures, including calcium regulators and mitochondrial function. Excessive activation of sympathetic nervous and renin–angiotensin systems may augment the risk of cardiac dysfunction [27][36]. Raised myocardial epinephrine concentration is associated with increased resting heart rate in rats with failing hearts [39][47]. However, it is unknown whether kaempferol has any effects on epinephrine level or β1-adrenoceptor in the heart that may somewhat contribute to its cardioprotective effects. Collectively, kaempferol can improve myocardial left ventricular systolic and diastolic function and prevent the development of arrhythmia. However, studies to date have only examined the effect of kaempferol on left ventricular function; no study has investigated its effect on right ventricular function. Future studies should focus on this aspect.4. Effects on Myocardial Calcium Regulation and Rhythm
Cardiac calcium regulation is closely linked to cardiac contraction. The sarcoplasmic reticulum is the main source of calcium for myocardial contraction [40][48]. Calcium regulatory proteins such as the sodium–calcium exchanger (NCX), Ca2+-ATPase, and sodium–potassium ATPase (Na+/K+-ATPase) govern the uptake and release of calcium ions across cell membrane [41][49]. A reduction in intracellular Na+ triggers NCX activity to bring more Na+ inside the cells in exchange for Ca2+ [41][49], while Ca2+-ATPase regulates the uptake of Ca2+ into cells and the sarcoplasmic reticulum (known as sarcoplasmic endoplasmic reticulum Ca2+-ATPase (SERCA)) [40][48]. Kaempferol (100 mg/kg) post-treatment for 45 days reversed the reduction in myocardial membrane-bound Na+/K+-ATPase, Ca2+-ATPase, and total ATPase activity in diabetic rats [16][26]. The findings suggest that kaempferol maintains cardiac calcium homeostasis and safeguards the integrity of the membrane under pathological conditions. The effects of kaempferol on other calcium regulators, such as NCX and ryanodine receptor type 2 (RyR2) for Ca2+ release and phospholamban, which is involved in Ca2+ uptake, are yet to be studied. Understanding these aspects could shed light on the mechanisms of action of kaempferol. Altered Ca2+ homeostasis can provoke cardiac arrhythmias, such as atrial fibrillation. In patients with metabolic syndrome, cardiac mitochondrial Ca2+ uniporter complex (MCUC) is downregulated, resulting in impaired mitochondrial Ca2+ uptake and leading to atrial fibrillation [42][50]. Kaempferol treatment protects against cardiac arrhythmia; it protects against the development of sinus node dysfunction [43][51] and ventricular arrhythmia in mice following LADCA ligation [42][50]. Kaempferol augments mitochondrial Ca2+ uptake [37][44][45][44,53,54] by restoring MCUC activity, and it abolishes atrial fibrillation in obese diabetic mice [42][50]. In addition, kaempferol may decrease the expression of mitochondrial Ca2+ uptake 1 (MICU1), a MCUC gatekeeper [46][55], leading to the augmented uptake of the ion by mitochondria (Figure 2).
Figure 2. Sites of action of kaempferol in myocardial injury. Akt, protein kinase B; APAF1, apoptotic protease activating factor 1; ARE, antioxidant response element; ATF6, activating transcription factor 6; CHOP, C/EBP homologous protein; CTGF, connective tissue growth factor; cyt. c, cytochrome c; eIF2, eukaryotic initiation factor 2α; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; GRP78, glucose regulatory protein 78; GSK3, glycogen synthase kinase-3; HO-1, heme oxygenase-1; IKB, inhibitor of κB kinase; NF-κB, nuclear factor kappa B; IKKα, inhibitor of NF-κB kinase α; IKKβ, inhibitor of NF-κB kinase β; IKKγ, inhibitor of NF-κB kinase γ; IRE1, inositol-requiring transmembrane kinase endoribonuclease-1α; JNK, c-Jun N-terminal kinase; Keap1, Kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; MCUC, mitochondrial Ca2+ uniporter complex; MICU1, mitochondrial Ca2+ uptake 1; MMP, matrix metalloproteinase; mPTP, mitochondrial permeability transition pore; NQO1, NAD(P)H dehydrogenase (quinone 1); Nrf2, nuclear factor erythroid 2 p45-related factor 2; p38, p38 MAPK; p53, p53 tumor suppressor gene; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; SIRT1, silent information regulator type 1; α-SMA, α-smooth muscle actin; Smad, small mothers against decapentaplegic; STING, stimulator of interferon genes; TFAM, mitochondrial transcription factor A; TGFβ, transforming growth factor β; TIMP; TNFα, tumor necrosis factor α; TNFR, tumor necrosis factor receptor; XBP1, X-box binding protein 1.
5. Effects on Cardiac Oxidative Stress and Inflammation
Oxidative stress and inflammation play a prominent role in various diseases, including cardiac disease [47][58]. ROS are implicated in oxidative stress [48][59] and inflammation [49][50][60,61]. Kaempferol protects the heart by reducing lipid peroxidation products (e.g., thiobarbituric acid reactive substance, malondialdehyde, conjugated diene, and lipid hydroperoxide); increasing antioxidant enzymes, namely superoxide dismutase, catalase, and glutathione peroxidase, in diabetic rats [33][51][52][5,31,38]; and decreasing pro-inflammatory cytokines (e.g., tumor necrosis factor α (TNFα) and interleukin 6) [12][19][6,8].
Nuclear factor erythroid 2 p45-related factor 2 (Nrf-2) is a transcription factor that modulates oxidative stress [53][64]. Upon activation, Nrf2 dissociates from its complex with Keap1 (Figure 2). Activated Nrf2 governs the antioxidant response by upregulating the expression of its downstream effectors, namely heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase (quinone 1) (NQO1) [53][54][64,65]. Upregulation of these antioxidant factors (Nrf2, HO-1, and NQO-1) by kaempferol was observed in various models of heart disease, including diabetic cardiomyopathy [6][13][11,13], high-glucose-induced cardiomyocyte injury [13], and Ang-II-induced cardiomyocyte hypertrophy [12][6]. Kaempferol also downregulates the Keap1 gene [6][11], which is suggestive of its ability to increase the activation of the antioxidant signaling pathway.
NF-κB is a principal inflammatory regulator that regulates chemokines and pro-inflammatory cytokines. To function, it needs to be activated prior to its translocation to the nucleus. This activation is strictly governed by the inhibitor of the κB kinase (IκBα and IκBβ) and inhibitor of the NF-κB kinase (IKKα and IKKβ) [55][66].
The MAPK and phosphoinositide 3-kinase/protein kinase B/glycogen synthase kinase-3β (PI3K/Akt/GSK3β) signaling pathways are involved in the modulation of oxidative stress and inflammation. MAPK has three subfamilies: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK (p38) [5][18].
