This paper explores advances in the detection and quantification of pharmaceutical substances, with a focus on the development of high-performance electrochemical sensors, particularly those based on chitosan, to address the need for sensitive and selective detection techniques in various applications.
This research explores advances in the detection and quantification of pharmaceutical substances, with a focus on the development of high-performance electrochemical sensors, particularly those based on chitosan, to address the need for sensitive and selective detection techniques in various applications.
- sensors
- drug
- pharmaceutical analysis
- carbon nanomaterials
1. Pharmaceutical Substance Detection and Quantification
Ensuring the accurate detection and measurement of pharmaceutical substances across various matrices is pivotal for human health, environmental protection, medication therapy monitoring, and the assurance of pharmaceutical product quality. To address contemporary needs and manage pharmaceutical substances effectively, the ongoing evolution of sensitive, selective, and economical detection techniques is imperative.
1.1. Traditional Analytical Methods
Historically, pharmaceutical detection has relied on established analytical techniques such as:
- Liquid chromatography-mass spectrometry (LC-MS) [1]
- Gas chromatography-mass spectrometry (GC-MS) [2]
- High-performance liquid chromatography (HPLC) [3]
- Capillary electrophoresis (CE) [4]
- Immunoassays [5]
These methods are recognized for their compound separation efficiency, sensitivity, and minimal sample volume needs. However, they can also pose challenges, including high costs, extended analysis time, and the necessity for specialized apparatus and expert personnel, especially in less-equipped or smaller laboratories[6][7].
1.2. Electrochemical Methods
Electrochemical techniques offer an alternative to traditional methods, boasting benefits such as cost-effectiveness, swift analysis, and limited sample preparation. These methods are not intended to supplant existing techniques but rather to augment the array of tools accessible to professionals in the domain.
Chitosan Based Electrochemical Sensors in Pharmaceutical Detection
Electrochemical sensors are gaining traction for pharmaceutical detection, attributed to their heightened sensitivity, selectivity, real-time monitoring, and the inherent benefits of electrochemical instrument miniaturization and portability. These devices operate by translating a biochemical process into an electric signal, typically gauging current or potential shifts stemming from a redox reaction on the sensor's surface [8]. The compactness and mobility of these instruments facilitate the creation of field-ready sensors, broadening their utility and reach [9]. Notwithstanding these advancements, challenges like reproducibility and enduring stability persist, topics further delved into in this review.
Chitosan, a biopolymer originating from chitin, is extensively researched for electrochemical sensor creation due to its distinctive attributes like biocompatibility, biodegradability, and expansive contact surface[10]. Chitosan (CTS) is amenable to functionalization and modification, rendering it a prime candidate for sensor development[11]. When amalgamated with nanomaterials, CTS augments the sensitivity, selectivity, and stability of electrochemical sensors. Carbon-based nanomaterials, such as graphene and carbon nanotubes, when integrated with CTS, elevate the electron transfer rate and boost electrocatalytic activity [12][13]. Metallic nanoparticles like gold and silver, in conjunction with CTS, amplify sensor sensitivity and selectivity [14]. Furthermore, conductive polymers, including polyaniline and polypyrrole, when paired with CTS, enhance sensor stability and sensitivity [6][15][16].
Detection and quantification of pharmaceutical substances in various matrices are essential for safeguarding human health and the environment, ensuring appropriate monitoring of medication therapies, and ensuring the quality of pharmaceutical products. Detection and quantification of pharmaceutical substances in various matrices are essential for safeguarding human health and the environment, ensuring appropriate monitoring of medication therapies, and ensuring the quality of pharmaceutical products.
Chitosan-based electrochemical sensors display remarkable adaptability across various applications. They've been successful in detecting anti-allergic substances, like chlorpheniramine maleate, phenylephrine, dextromethorphan, and cetirizine, due to the prevalence of allergic diseases [17]. Additionally, CTS-based sensors are widely employed in detecting pharmaceutical substances with diverse indications, such as paracetamol, clindamycin, diclofenac, propranolol, metformin, and fluconazole. They also find utility in identifying metabolites like cholesterol, uric acid, xanthine, hypoxanthine, p53 protein, and purines as potential biomarkers . These sensors contribute to addressing the global concern of narcotics misuse and dependence, with applications in detecting prohibited substances in biological samples and forensic investigations [18]. The The continuous development of new sensitive, selective, and cost-effective detection methods is necessary to meet current demands and ensure proper management of pharmaceutical substances in modern society.continuous development of new sensitive, selective, and cost-effective detection methods is necessary to meet current demands and ensure proper management of pharmaceutical substances in modern society.
2. Chitosan (CTS): Origin, Structure, and Properties
2.1. Source of Chitosan: Chitin
Chitin stands as the second most prevalent natural polymer, surpassed only by cellulose. It serves as a structural component in fungal cell walls, certain algae, and the exoskeletons of crustaceans like shrimp, crabs, and lobsters [19].
Production and Chemical Transformation of CTS
Chitosan emerges from the deacetylation of chitin. This transformation entails exposing chitin to alkaline solutions or specific enzymes that facilitate the removal of acetyl groups (-COCH3). The resultant polymer, CTS, possesses a greater concentration of amino groups (-NH2) compared to chitin. A defining trait of CTS is its amine group with a pKa value of 6.5, rendering its surface positively charged. This charge enables CTS to engage with negatively charged entities, such as nucleic acids or other biological molecules, a property harnessed in drug delivery systems where CTS serves as a drug or genetic material conduit [20].
Structural Illustration
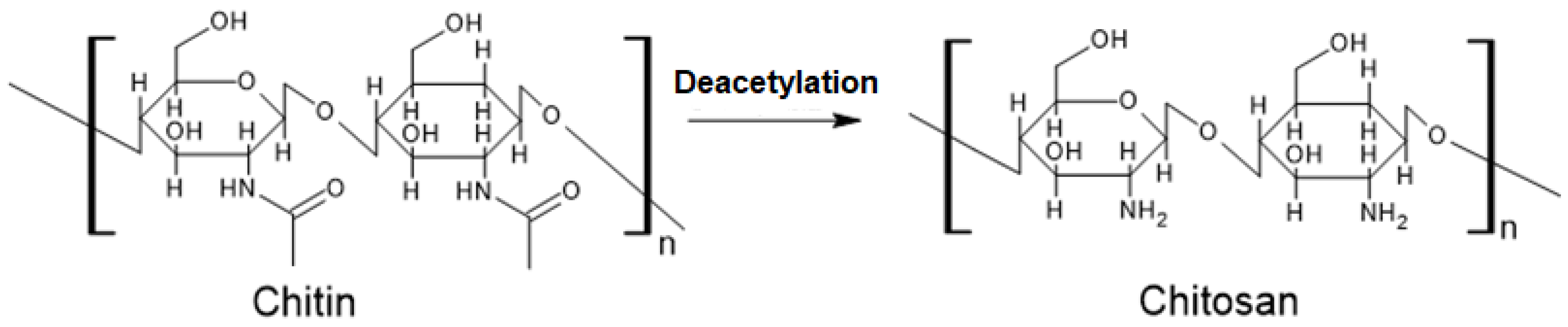
Figure 1:. Depiction of CTS's formation via chitin deacetylation.
2.2. Modifications and Functionalities of CTS
CTS's structure can be tailored both chemically and physically to introduce diverse functional groups, including amino, carboxyl, or thiol groups. These modifications can enhance sensor electrochemical performance or facilitate biomolecule immobilization. Blending CTS with polymers like polyvinyl alcohol or polyethylene glycol can amplify its film-forming and mechanical attributes [6].
2.3. Applications and Studies on CTS-based Sensors
Research has delved into the multifaceted applications of CTS in sensor technology. A notable review by Suginta, Khunkaewla, and Schulte offers an exhaustive exploration of sensors utilizing chitin and CTS, shedding light on the innovations and applications of these materials in sensor technology [21]. The inherent modifiability of CTS underscores its potential in crafting electrochemical sensors with heightened sensitivity and selectivity . Beyond its physical attributes, CTS's chemical properties, such as its capacity to form stable complexes with metal ions, enable the integration of diverse nanomaterials like carbon nanotubes and metallic nanoparticles [22]. Its expansive contact surface facilitates efficient biomolecule immobilization, crucial for biosensor creation [23]. Additionally, CTS's antimicrobial properties [24] position it as a valuable tool for detecting pathogenic microorganisms [25] [26]. Its adaptability through chemical alterations and its versatility in various formulations amplify its potential applications [12][27].
The inherent properties of CTS, encompassing biocompatibility, biodisponibility, solubility, cationic nature, expansive contact surface, antimicrobial activity, and functional adaptability, render it a prime candidate for crafting electrochemical sensors, especially for pharmaceutical detection. These attributes collectively contribute to the creation of cost-effective, high-performance sensors [28].
3. Electrochemical Sensors: Principles and Applications
3.1. Introduction to Electrochemical Sensors
Electrochemical sensors have become indispensable in diverse sectors, notably in pharmaceutical detection. Their operational principles are foundational to grasping their utility and potential applications. This segment delves into the principles steering the function of CTS-based electrochemical sensors.
3.2. Advantages of Electrochemical Sensors
These sensors are lauded for their heightened sensitivity, selectivity, and swift response times. Their cost-effectiveness and user-friendliness further bolster their appeal. Research has underscored their versatility, with studies showcasing their efficacy in detecting heavy metal ions in water [28] and volatile organic compounds in air [29]. Their potential in environmental monitoring, especially in resource-constrained regions, has been highlighted [30].
Technological Advancements
Innovations in materials science and nanotechnology have ushered in advanced electrochemical sensors with superior performance. The integration of nanomaterials has been shown to augment the sensitivity and selectivity of these sensors [31].
3.3. Operational Principles
At the heart of an electrochemical sensor lies the interaction between the analyte and the sensor's active layer, culminating in signal transduction to the recording apparatus. These sensors typically comprise electrochemical cells, with the working electrode's active material, the analyte, the reaction medium, and the electrode set being integral components. The sensors register changes in current or potential due to redox reactions on their surface. They can be broadly categorized into potentiometric and amperometric types, as per IUPAC definitions [32].
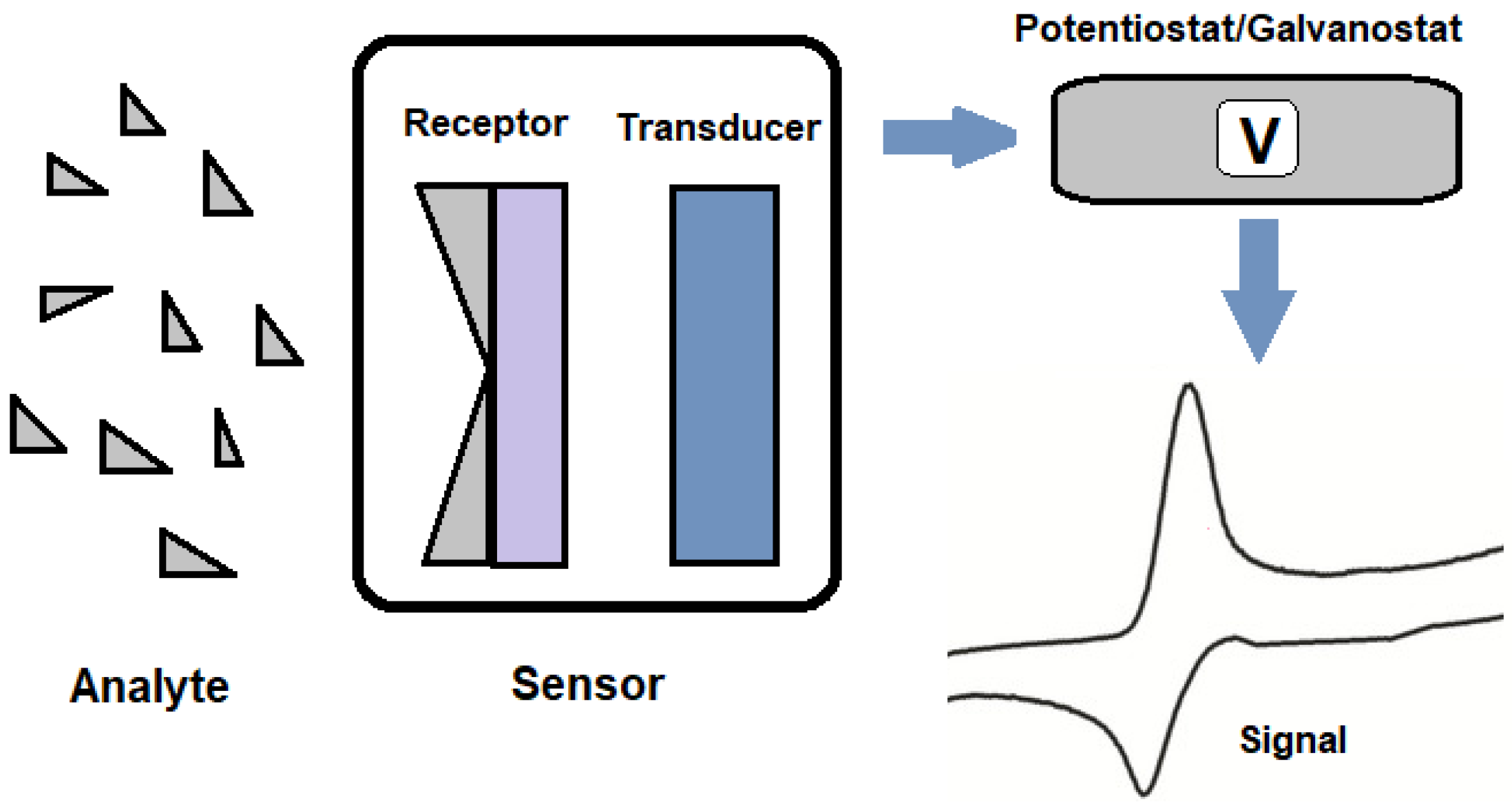
Figure 2:. Electrochemical sensor schematic representation.
3.4. Sensor Components and Design
The sensor's efficacy hinges on a myriad of factors, encompassing electrode type, electroactive species nature, electrolyte solution composition, and sensor design specifics. Three pivotal electrode types are recognized: working, reference, and auxiliary electrodes[33]. The working electrodes are where pivotal electrochemical reactions transpire, while reference electrodes offer a stable potential, ensuring measurement precision. Auxiliary electrodes, in tandem with working electrodes, facilitate electron flow, engendering signal generation [32][34].
3.5. Material Selection and Sensor Performance
The choice of working electrode material can influence the sensor's sensitivity, selectivity, and stability. Common materials encompass noble metals and conducting polymers [13][35][36]. Enhancements in sensitivity and selectivity can be achieved by modifying the active surface. CTS, for instance, offers a stable platform for biomolecule immobilization [37]. The inclusion of nanomaterials can further refine electrochemical performance [38].
3.6. Sensor Types and Mechanisms
-
Potentiometric Sensors: Measure potential differences between working and reference electrodes, correlating with target analyte concentration. CTS's stability and biocompatibility amplify the sensor's performance.
-
Amperometric Sensors: Gauge current from an analyte's electrochemical reaction. CTS serves as a matrix for enzyme immobilization, facilitating accurate current measurements.
-
Impedimetric Sensors: Operate by gauging impedance in the presence of an analyte. CTS-analyte interactions can induce impedance shifts, enabling specific detections[13][35][36].
3.7. Challenges and Future Directions
While CTS-based sensors present myriad advantages, it's crucial to recognize their limitations, such as specificity in intricate sample matrices and scalability. Balancing advanced design with practicality remains a challenge. The robustness and reliability of these sensors in real-world scenarios necessitate further exploration. The incorporation of biomaterials like CTS introduces complexities that warrant meticulous attention to harness the full potential of electrochemical sensors. The future trajectory for these sensors encompasses innovative methodologies, sustainability considerations, and fostering interdisciplinary collaborations and standardizations.
References
- Beccaria, M.; Cabooter, D. Current Developments in LC-MS for Pharmaceutical Analysis. Analyst 2020, 145, 1129–1157.
- Deng, A.; Himmelsbach, M.; Zhu, Q.-Z.; Frey, S.; Sengl, M.; Buchberger, W.; Niessner, R.; Knopp, D. Residue Analysis of the Pharmaceutical Diclofenac in Different Water Types Using ELISA and GC−MS. Environ. Sci. Technol. 2003, 37, 3422–3429.
- Yabré, M.; Ferey, L.; Somé, I.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23, 1065.
- Krait, S.; Konjaria, M.; Scriba, G.K.E. Advances of Capillary Electrophoresis Enantioseparations in Pharmaceutical Analysis (2017–2020). Electrophoresis 2021, 42, 1709–1725.
- Soleymani, J.; Golsanamluo, Z. Advanced Materials for Immunosensing of Pharmaceutical and Drug Compounds. ImmunoAnalysis 2021, 1, 5.
- Annu; Raja, A.N. Recent Development in Chitosan-Based Electrochemical Sensors and Its Sensing Application. Int. J. Biol. Macromol. 2020, 164, 4231–4244.
- Seger, C. Usage and Limitations of Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS) in Clinical Routine Laboratories. Med. Wochenschr. 2012, 162, 499–504.
- Mollarasouli, F.; Zor, E.; Ozcelikay, G.; Ozkan, S.A. Magnetic Nanoparticles in Developing Electrochemical Sensors for Pharmaceutical and Biomedical Applications. Talanta 2021, 226, 122108
- Federica Mariani; Isacco Gualandi; Wolfgang Schuhmann; Erika Scavetta; Micro- and nano-devices for electrochemical sensing. Microchim. Acta 2022, 189, 1-31.
- Prabhu, A.; Crapnell, R.D.; Eersels, K.; Van Grinsven, B.; Kunhiraman, A.K.; Singla, P.; McClements, J.; Banks, C.E.; Novakovic, K.; Peeters, M. Reviewing the Use of Chitosan and Polydopamine for Electrochemical Sensing. Curr. Opin. Electrochem. 2022, 32, 100885.
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848.
- Shukla, S.K.; Lavon, A.; Shmulevich, O.; Ben-Yoav, H. The Effect of Loading Carbon Nanotubes onto Chitosan Films on Electrochemical Dopamine Sensing in the Presence of Biological Interference. Talanta 2018, 181, 57–64.
- Yusoff, N. Chapter 7—Graphene–Polymer Modified Electrochemical Sensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Pandikumar, A., Rameshkumar, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–186. ISBN 978-0-12-815394-9.
- Santos, A.M.; Wong, A.; Fatibello-Filho, O. Simultaneous Determination of Salbutamol and Propranolol in Biological Fluid Samples Using an Electrochemical Sensor Based on Functionalized-Graphene, Ionic Liquid and Silver Nanoparticles. J. Electroanal. Chem. 2018, 824, 1–8.
- Karrat, A.; Amine, A. Recent Advances in Chitosan-Based Electrochemical Sensors and Biosensors. J. Chem. Environ. Res. 2020, 7, 66–93.
- Zahed, F.M.; Hatamluyi, B.; Lorestani, F.; Es’haghi, Z. Silver Nanoparticles Decorated Polyaniline Nanocomposite Based Electrochemical Sensor for the Determination of Anticancer Drug 5-Fluorouracil. J. Pharm. Biomed. Anal. 2018, 161, 12–19.
- Anuja S. Rajpurohit; Ashwini K. Srivastava; Simultaneous electrochemical sensing of three prevalent anti-allergic drugs utilizing nanostructured manganese hexacyanoferrate/chitosan modified screen printed electrode. Sensors Actuators B: Chem. 2019, 294, 231-244.
- Ruilin Zhang; Kaixin Fu; Fangyuan Zou; Huiping Bai; Genlin Zhang; Feng Liang; Qingju Liu; Highly sensitive electrochemical sensor based on Pt nanoparticles/carbon nanohorns for simultaneous determination of morphine and MDMA in biological samples. Electrochimica Acta 2021, 370, 137803.
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; pp. 5–18. ISBN 978-9-81137-318-3.
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical Biosensor Applications of Polysaccharides Chitin and Chitosan. Chem. Rev. 2013, 113, 5458–5479.
- Silva, S.B.; Batista, G.L.; Santin, C.K. Chitosan for Sensors and Electrochemical Applications. In Chitin and Chitosan; Broek, L.A.M., Boeriu, C.G., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 461–476. ISBN 978-1-119-45043-6.
- Petrucci, R.; Pasquali, M.; Scaramuzzo, F.A.; Curulli, A. Recent Advances in Electrochemical Chitosan-Based Chemosensors and Biosensors: Applications in Food Safety. Chemosensors 2021, 9, 254.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Dhanavel, S.; Manivannan, N.; Mathivanan, N.; Gupta, V.K.; Narayanan, V.; Stephen, A. Preparation and Characterization of Cross-Linked Chitosan/Palladium Nanocomposites for Catalytic and Antibacterial Activity. J. Mol. Liq. 2018, 257, 32–41.
- Gill, A.A.S.; Singh, S.; Nate, Z.; Chauhan, R.; Thapliyal, N.B.; Karpoormath, R.; Maru, S.M.; Reddy, T.M. A Novel Copper-Based 3D Porous Nanocomposite for Electrochemical Detection and Inactivation of Pathogenic Bacteria. Sens. Actuators B Chem. 2020, 321, 128449.
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent Progress in the Structural Modification of Chitosan for Applications in Diversified Biomedical Fields. Eur. Polym. J. 2018, 109, 402–434.
- Shi, Y.; Jiao, H.; Sun, J.; Lu, X.; Yu, S.; Cheng, L.; Wang, Q.; Liu, H.; Biranje, S.; Wang, J.; et al. Functionalization of Nanocellulose Applied with Biological Molecules for Biomedical Application: A Review. Carbohydr. Polym. 2022, 285, 119208.
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, Label-Free Voltammetric Determination of Norfloxacin Based on Molecularly Imprinted Polymers and Au Nanoparticle-Functionalized Black Phosphorus Nanosheet Nanocomposite. J. Hazard. Mater. 2022, 436, 129107.
- Li, G.; Wu, J.; Qi, X.; Wan, X.; Liu, Y.; Chen, Y.; Xu, L. Molecularly Imprinted Polypyrrole Film-Coated Poly(3,4-Ethylenedioxythiophene): Polystyrene Sulfonate-Functionalized Black Phosphorene for the Selective and Robust Detection of Norfloxacin. Mater. Today Chem. 2022, 26, 101043.
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Jatoi, A.S. Advanced Growth of 2D MXene for Electrochemical Sensors. Environ. Res. 2023, 222, 115279.
- Wang, L.; Pagett, M.; Zhang, W. Molecularly Imprinted Polymer (MIP) Based Electrochemical Sensors and Their Recent Advances in Health Applications. Sens. Actuators Rep. 2023, 5, 100153.
- Wang, J. Analytical Electrochemistry; Wiley: Hoboken, NJ, USA, 2006.
- Yan, K.; Karthick Kannan, P.; Doonyapisut, D.; Wu, K.; Chung, C.; Zhang, J. Advanced Functional Electroactive and Photoactive Materials for Monitoring the Environmental Pollutants. Adv. Funct. Mater. 2021, 31, 2008227.
- Simões, F.R.; Xavier, M.G. Electrochemical Sensors. In Nanoscience and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–178. ISBN 978-0-323-49780-0.
- Pingarrón, J.M.; Labuda, J.; Barek, J.; Brett, C.M.A.; Camões, M.F.; Fojta, M.; Hibbert, D.B. Terminology of Electrochemical Methods of Analysis (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 641–694.
- Aralekallu, S.; Sannegowda, L.K. Chapter 25—Metal Nanoparticles for Electrochemical Sensing Applications. In Handbook of Nanomaterials for Sensing Applications; Hussain, C.M., Kailasa, S.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 589–629. ISBN 978-0-12-820783-3.
- Lee, J.F.; Thirumavalavan, M. A Short Review on Chitosan Membrane for Biomolecules Immobilization. J. Mol. Genet. Med. 2015, 9, 1000178.
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360.
- Federica Mariani; Isacco Gualandi; Wolfgang Schuhmann; Erika Scavetta; Micro- and nano-devices for electrochemical sensing. Microchim. Acta 2022, 189, 1-31.
- Anuja S. Rajpurohit; Ashwini K. Srivastava; Simultaneous electrochemical sensing of three prevalent anti-allergic drugs utilizing nanostructured manganese hexacyanoferrate/chitosan modified screen printed electrode. Sensors Actuators B: Chem. 2019, 294, 231-244.
- Ruilin Zhang; Kaixin Fu; Fangyuan Zou; Huiping Bai; Genlin Zhang; Feng Liang; Qingju Liu; Highly sensitive electrochemical sensor based on Pt nanoparticles/carbon nanohorns for simultaneous determination of morphine and MDMA in biological samples. Electrochimica Acta 2021, 370, 137803.
