Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ugo Rogo and Version 2 by Fanny Huang.
Embryo rescue (ER) techniques are among the oldest and most successful in vitro tissue culture protocols used with plant species. ER refers to a series of methods that promote the development of an immature or lethal embryo into a viable plant.
- embryo rescue
- plant breeding
- immature embryo
- hybridization
- ploidy levels
1. Introduction
In Angiosperm, the seed is produced by double fertilization. The male gametophyte sperm cell fuses a central diploid cell derived from the union of the two female polar nuclei, creating a triploid endosperm. Simultaneously, the second male sperm cell fuses with the egg cell to produce the diploid zygote. The resulting embryo (2n) and endosperm (3n) continue to develop within the seed coat derived from the maternal tissues of the egg [1]. In most dicot plants after fertilization, the nucleus of the zygote moves to the apical pole, and the zygote divides asymmetrically to produce a small apical cell that will generate the entire embryo apart from the suspensor and root cap, which are derived from the larger basal cell. The cell division generates a mature embryo organized with an apical–basal polarity and radial concentric tissue layers perpendicular to the axis of symmetry. The apical end is formed by the primary shoot apical meristem (SAM), flanked by one or two cotyledons, while the primary root apical meristem (RAM) is located at the basal end [2]. From the early stages of pattern formation and morphogenesis, the stem cell pools, which are essential for virtually unlimited post-embryonic growth, are placed in the RAM and SAM [3]. Conversely, storage reserves accumulate during the next stage of maturation to prepare the embryo for developmental arrest [4]. In most dicot plants, the endosperm is temporary and, therefore, consumed by the embryo during seed maturation, leaving only a peripheral aleurone-like cell layer next to the seed coat, surrounding the mature embryo [5]. Monocotyledonous zygotes, such as maize, rice, and wheat, also exhibit polarity with the nucleus located at the apical pole. The first transverse division of the zygote produces a two-cell embryo, where the basal cell transforms into a large vesicular cell, while the continuous division of the apical cell gives rise to the quadrant, octant, and dermatogenous stages (proembryos stages) [6]. During the later transitional embryonic stage, which in maize occurs about 7–8 days after pollination (DAP), the adaxial–abaxial axis of the embryo becomes evident. The coleoptile primordium starts protruding from the adaxial region, while the scutellar region develops from the abaxial side. The coleoptile protects the development of the SAM, while the scutellum is analogous to the cotyledon in dicot species. The SAM develops on the adaxial side and produces several embryonic leaves during seed development, while the differentiation of the RAM defines the basal pole of the embryo. In rice, the embryo reaches its mature shape when organ differentiation is complete at 7–8 DAP, although morphogenesis continues as the embryo enlarges. The SAM and RAM are enclosed by the protective coleoptile and coleorhiza, respectively. In grasses, the early vegetative stages of embryonic seedlings are incorporated into the embryo before entering dormancy [7].
Many physical, chemical, and genetic factors influence the development of the zygotic embryo [8]. Disruptions in these factors can result in abnormalities and, in the worst-case scenario, embryo abortion [9][10][9,10].
Embryo rescue (ER) is a set of techniques commonly used to rescue immature/mature-lethal embryos, and hybrid embryos generated from interspecific and intergeneric crosses unable to survive in vivo or during traditional plant breeding practices [11][12][13][14][15][16][17][18][19][11,12,13,14,15,16,17,18,19]. The procedure involves excising immature or lethal embryos and culturing them in vitro on a specific nutrient culture medium. The developmental differences between dicots and monocots need to be considered when attempting to recover immature embryos. Nutritional species-specific requirements and the assessment of parameters about growth conditions deserve particular attention to distinguish what happens in the normal embryonic development of dicot and monocot embryos.
During the domestication of plants, many genes controlling resistance to biotic and abiotic stresses were lost. The initial goals of the domestication process were the modification of important traits, such as plant architecture, seed dormancy, fruit and seed size, absence of antinutrients, thorns and waxes, compactness of the ears, absence of seed dispersal, and synchronous fruit ripening. This has led to the phenomenon known as domestication syndrome, where plant survival becomes entirely dependent on human care [20]. Unfortunately, the stress-resistant characteristics of wild ancestor species were often overlooked. However, wild ancestors of crops and/or related wild species constitute a large reservoir of genes that can be introduced into cultivated crops through interspecific and intergeneric hybridization [21]. The rediscovery of Mendelian laws laid the foundation for genetic improvement based on the inheritance of characteristics. However, crossing often requires overcoming physiological and genetic barriers, such as fertilization incompatibility, embryo abortion, and seed dormancy. ER and direct in vitro pollination, such as pollinating stigmas or pistils and opened ovaries or ovules, have proven advantageous in overcoming embryo abortion and pre-zygotic incompatibility barriers, respectively [22]. In vitro pollination techniques have yielded the best results in species with large ovaries containing numerous ovules, including families such as Brassicaceae, Caryophyllaceae, Papaveraceae, Primulaceae, and Solanaceae [23][24][23,24]. ER has been instrumental in recovering haploid, dihaploid, doubled haploid (DH), polyploid embryos, addition lines, and substitution lines resulting from interspecific or intergeneric crosses. ER techniques have been also used to shorten the reproductive cycle, propagate rare plants, study the physiology of seed germination, understand embryo nutrition, and study embryo morphogenesis from the zygote to cotyledon stage [12][17][19][25][26][27][28][29][30][31][12,17,19,25,26,27,28,29,30,31] (Figure 1).
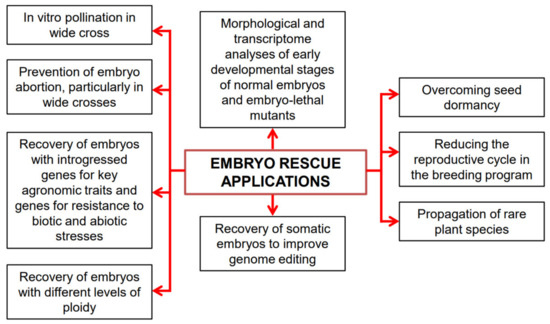
Figure 1. A diagrammatical representation of embryo rescue (ER) applications.
2. The Origins of Embryo Rescue: A Brief Historical Overview
Charles Bonnet (1720–1793) documented ER for the first time in the 18th century. He achieved remarkable success by excising mature embryos of Phaseolus vulgaris and Fagopyrum and transplanting them into the soil where they could grow [14]. Later, many scientists started assessing the process with various nutrient media. From 1890 to 1904, ER was evaluated with nutrient media containing salt and sugars along with tissue culture techniques. In 1904, E. Hanning successfully introduced mature embryos into the in vitro tissue culture of some Brassicaceae species (i.e., Raphanus sativus, R. landra, R. caudatus, and Cochlearia danica), obtaining seedlings on a saline medium supplemented with sugar. However, he found the problem that in vitro embryo culture originated small and faint seedlings compared to those raised in vivo [32][33][32,33]. From these experiments, Hanning focused on the necessity of a high osmotic concentration in the culture medium since the embryos were unable to grow in media lacking sucrose as a carbon source, and the essentiality of an adequate source of nitrogen to allow seedling development from embryos [12][32][12,32].
The culture method was used by Brown [34] to assess the efficacy of various organic nitrogenous compounds in promoting the growth of isolated barley (Hordeum vulgare) embryos nourished in a mineral saline medium supplemented with sucrose. These experiments demonstrated that amino acids, such as aspartic acid, glutamic acid, and asparagine, served as superior nitrogen sources, leading to increased dry weight and nitrogen content of cultured embryos. Other studies have shown the favorable action of reserve tissues, such as endosperm and cotyledons, in embryo development, although their indispensability was not specifically established [35]. Nevertheless, the reduced growth of Phaseolus lanatus embryos deprived of cotyledons and cultured in distilled water solidified with agar was significantly improved by adding glucose or an extract containing reducing sugar from cotyledons of germinated seeds [36]. Andronescu [37] observed the stunted growth of maize (Zea mays) seedlings from embryos lacking scutellum, demonstrating the importance of this organ in the absorption of essential nutrients for the development of monocot embryos.
In 1924, Kurt Dieterich was a precursor in testing the possibility of embryo culture both in mature and immature explants [38]. His experiments involved culturing embryos from species of various families, including Solanaceae, Linaceae, Brassicaceae, Polygonaceae, Asteraceae, Cucurbitaceae, and Poaceae, revealing differences in nutrient requirements, such as carbon and nitrogen sources, between immature and mature embryos.
In vivo, embryos derived from interspecific or intergeneric crosses are often underdeveloped and lethal. Frequently, crosses between plants with incompatible genomes lead to early embryo abortion. Often, ER allows the growth of these embryos with reduced viability until development into mature plants. The first application of the in vitro culture of zygotic immature embryos from interspecific cross dates to 1925, when Friedrich Laibach used this technique to prevent embryonic abortion in Lilium perenne × L. austriacum cross [39]. Laibach’s experiments were fundamental because they gave the initial impetus to the use of interspecific and intergeneric hybridization in genetic improvement, even between species in which the nature of incompatibility did not allow the enormous genetic variability present in wild species to be exploited.
3. The Embryo Rescue Techniques
3.1. Pre-Zygotic Barriers in Hybridization and Techniques to Overcome Them
Intraspecific, interspecific, and intergeneric hybridizations are important reproductive mechanisms that have contributed to plant speciation and plant breeding [21]. These crosses allow the introgression of advantageous genes from wild species to improve cultivated crops. However, pre- and post-mating reproductive barriers often hinder the application of hybridization techniques in genetic improvement, especially when phylogenetically distant species are involved [40]. Post-mating reproductive barriers can be pre-zygotic and post-zygotic because incompatibility reactions occur before (pre-zygotic) and after (post-zygotic) fertilization, respectively. Notably, the positive fate may depend on the crossing direction for each hybridization.
In the pre-zygotic type, the incompatibility reaction often results in the failure of pollen germination, pollen tube growth, or pollen tube penetration into the ovule, which may occur at various levels in different tissues, such as the stigma, style, or ovary [40][41][42][40,41,42].
To overcome pre-zygotic barriers, cut-style or graft-on-style techniques, application of pollen mixture from several species, placenta pollination, and in vitro ovule pollination have been used [43][44][45][43,44,45]. The techniques to be applied depend on the type of genetic barrier (i.e., incompatibility) that prevents fertilization.
To conduct in vitro fertilization, bringing fully functioning male and female gametophytes into contact and establishing optimal culture conditions to ensure successful gamete fusion is crucial. In pollination methods in vitro, the reproductive organs (stigma and anthers) are isolated, followed by controlled fusion of the male and female gametes. In addition, it is necessary to set up a culture medium that allows the zygote to develop to full maturity, followed by seed germination.
The graft-on-style method is useful when pollen germinates on the stigma but an incompatibility reaction occurs between pollen and style. This type of incompatibility, known as gametophytic incompatibility, causes the pollen tubes to stop and burst after traveling approximately one-third of the length of the style. In this case, the pollination of the entire gynoecium in vitro would not yield fruitful results, as all pollen tubes would be halted at the early part of the style, even under in vitro conditions. To overcome this challenge, the grafting technique is employed, wherein an incompatible style is replaced with a compatible one. This allows the pollen tube to follow its normal growth with the compatible style grafted into the ovary. Alternatively, in species with this type of genetic incompatibility, one proceeds by either shortening the style and micrografting the stigma at the base of the style or by directly depositing pollen on the ovule.
If incompatibility is sporophytic, the recognition substances are deposited in the exine of the pollen during gametogenesis, and the incompatibility reaction occurs at the level of the stigma. Therefore, for the purpose of promoting in vitro fertilization, delivery of pollen on the ovary or ovules may be preferable. Moreover, even placement of germinated pollen grains directly on the decapitated style of the stigma may be equally interesting [27][46][27,46].
3.2. Post-Zygotic Barriers in Hybridization and Techniques to Overcome Them
When the incompatibility is post-zygotic, the culture of immature embryos may be required. Interspecific and intergeneric hybridizations hinder many post-zygotic barriers [47][48][49][47,48,49], the mechanisms of which are not fully understood yet, and these aspects further complicate the adoption of the best ER techniques. Nevertheless, in vitro culture of immature embryos has emerged as the most widely employed technique, demonstrating remarkable practical success in interspecific and intergeneric hybrids production. These techniques have enabled the transfer of beneficial genes from wild to cultivated species. In higher plants, embryo development is closely linked to the presence of a well-formed endosperm. While the endosperm can develop without the embryo, the absence of endosperm results in early embryo abortion [40][42][50][40,42,50]. Failure to form endosperm commonly accompanies interspecific and intergeneric crosses, and this phenomenon is often present in embryo-lethal mutants. In flowering plants, the endosperm plays a crucial role in coordinating nutrient and hormone supply from maternal tissues to the embryo [51]. Several mechanisms have been proposed to explain hybridization barriers associated with abnormal endosperm differentiation. These include the endosperm equilibrium number (maternal: paternal ratio of 2:1) [52], the difference between the activation and response values expressed by the polar nuclei activation index [53], and genomic imprinting, with the emerging view that it largely influences parental genome dosage [45][54][45,54]. Under these circumstances, embryos frequently abort during their development, and ER procedures have proven useful for overcoming these barriers across a wide range of plant species.
Successful results in immature embryo survival depend on the technique adopted (e.g., culture in the ovary, ovule, or embryo). Equally important are the following aspects: the excision procedure, the preservation of embryo integrity, the protocol of sterilization methods, the culture medium composition, and the environmental conditions encompassing light intensity, quality, and temperature. Intrinsic factors, such as embryo size and developmental stage, also play an important role [12][19][25][28][29][55][12,19,25,28,29,55].
In embryo culture, the rescue of a very young embryo is more challenging than an already differentiated one. Ideally, it is more advantageous to recover the immature embryo at a later stage, often referred to as the “autotrophic phase” by some authors [56]. Hence, the embryo is excised from the plant when it reaches the maximum possible development within the constraints imposed by the absence of the endosperm. Typically, excision occurs within the first two weeks after pollination.
Embryo recovery at an early stage of their development, such as the globular or heart stage, may be preferable in certain interspecific combinations. This is because reaching a more advanced stage of development, such as torpedo or cotyledon, often leads to an abortion of the embryo in vivo that is no longer recoverable. However, working with these early embryos presents greater challenges because great is the risk of damaging them during excision, and complex are their nutritional requirements, particularly for proembryos [11].
To overcome these difficulties, the technique of ovule culture allows for the collection of immature embryos within their ovules. This involves in vitro culture of the fertilized egg cell for several days (e.g., one week) followed by the excision of the immature embryo from the cultured ovule. The excised embryo is then transferred to a substrate where it can complete its development [57][58][59][57,58,59]. This method produced new genomic combinations in species, genera, and families of dicot and monocot, for instance, Nicotiana [60], Gossypium [61], Brassica [62][63][62,63], Helianthus [58], Tulipa [64], Lilium [65], Vanilla [66], Rhododendron subgenera or sections [67], and Vaccinium [68][69][70][68,69,70], among others.
Generalizing the methods and media used in various in vitro culture techniques is inherently challenging. Each class (dicots or monocots), family, species, and genotype, as well as embryo derived from different hybridizations, requires the setup of specific methodologies to create the most suitable culture medium. This involves several aspects, such as salt concentrations, carbohydrates, vitamins, plant growth regulators (PGRs), and pH, as well as the exploration of proper environmental culture conditions, including temperature, light, and photoperiod. It is most likely that the optimized conditions are species-specific and, therefore, unsuitable for immature embryos of other species or those derived from different crosses. Moreover, dicot species frequently require a multi-step approach, starting from ovule culture to ER, unlike monocot species. This relates to the different types of development that characterize the embryos of dicots compared to monocots. Below, two examples of culturing ovules from phylogenetically distant dicot species are reported.
The in vitro ER technique was employed to obtain embryos from interspecific hybridizations between sunflower and other Helianthus species. The embryos were cultured for 7 days in ovules on artificial agarized media [58]. During this culture period, 51 to 84% of embryos of five sunflower genotypes developed to the vascular stage, comparable to greenhouse-grown plants. The survival and development of ovule-grown sunflower embryos were not affected by the salt composition of the four culture media tested: B5 and B5S [71], MS [72], and NN [73], nor did they show any benefit from high sucrose levels (90 or 120 g L−1) [58].
The ovule culture technique was utilized to enhance the survival of hybrid embryos between H. annuus × H. maximiliani Schrad. Approximately, 52% of the hybrid embryos exhibited viability when sunflower ovules were grown on standard media, indicating that the ovules provided the necessary requirements for the early stages of hybrid embryo development. Ovules were isolated and cultured for 14 days on fresh media with the same composition. About 50% of H. annuus embryos and 25% of H. annuus × H. maximiliani hybrid embryos germinated into seedlings. Six H. annuus × H. maximiliani seedlings were transferred to the greenhouse until flowering. All plants showed intermediate traits inherited from both parents [58].
In another study, Momotaz et al. [59] conducted intergeneric hybridizations to introduce genetic variability in crucifer crops. Seven species of Brassica (i.e., B. campestris var. trilocularis, B. campestris var. pekinensis, B. nigra, B. oleracea var. capitata, B. oleracea var. alboglabra, B. juncea var. napiformis, and B. carinata) and three species of Sinapis (i.e., S. alba, S. arvensis, and S. turgida) were used; reciprocal crosses were also made. In these hybridizations, ER played an essential role in overcoming post-zygotic barriers. Two methods were employed for hybrid plant development: in vitro ovary–ovule culture and direct ovule culture. However, the ovule culture showed better responses in terms of hybrid plant rescued. In this method, young fruits were harvested 15–20 DAP, and ovules were cultured on solidified B5 medium [71] supplemented with 2.5 mg L−1 1-Naphthaleneacetic acid (NAA), 2.5 mg L−1 N6-Furfuryladenine (kinetin), and 150 mL L−1 coconut milk [74]. Both callus formation and direct germination of embryos were observed from ovules. Calli were transferred to an MS medium [72] supplemented with 0.5 mg L−1 NAA and 2.5 mg L−1 kinetin. Germinated embryos were directly cultured on a hormone-free MS medium. The cultures were maintained at 25 ± 2 °C with a 16 h photoperiod. Seedlings were multiplied in vitro by growing shoots and nodal segments on MS medium, and they were later transplanted in the greenhouse on suitable soil. Specifically, embryos were obtained from the crosses between B. campestris var. trilocularis × S. turgida, B. campestris var. pekinensis × S. arvensis, S. arvensis × B. campestris var. pekinensis, B. oleracea var. alboglabra × S. alba, B. oleracea var. alboglabra × S. turgida, B. carinata × S. alba, B. carinata × S. arvensis, and B. carinata × S. turgida. The highest efficiency was detected in B. carinata × S. arvensis (11.5%) with 29 plants. Reciprocal crosses resulted in no hybrid, except with the combination of S. arvensis × B. campestris var. pekinensis. Morphological characteristics, chromosome number, and isoenzyme analyses confirmed the hybrid nature of the plants. Among the six combinations, four were identified as true hybrids, one as sesquidiploid, and one as a false hybrid [59].
To overcome the challenges associated with culturing young embryos, an interesting technique provides the relocation of hybrid embryos to a normally developing cellular endosperm dissected from a normal ovule of the parents or a third species, which serves as “nurse tissue”. The embryos and endosperm tissue are then transferred to the surface of the culture medium to activate the in vitro growth of the hybrid embryos [75]. Williams et al. [75] implemented a genetic improvement program involving some types of interspecific crosses, such as Trifolium ambiguum × T. repens, T. ambiguum × T. hybridum, T. repens × T. uniflorum, Ornithopus sativus × O. compressus, O. pinnatus × O. sativus, and Lotus pedunculatus × L. corniculatus. The “nurse tissue” technique was essential for the cross O. pinnatus × O. sativus hybrids to overcome defective endosperm development and for the cross T. ambiguum × T. hybridum, which resulted in completely sterile progeny. An interesting feature of the embryo culture on normal endosperm was the high success rate in the apparently normal and complete development of the hybrid embryos. In fact, although at least 100 putative hybrid proembryos were grown per cross, only a few seedlings were transferred into the soil and reached maturity.
The globular embryos of Capsella bursa-pastoris, smaller than 50 μm in size, showed higher survival rates when grown in ovules cultured in vitro than when individually inoculated onto the medium [76]. Monnier [77] proposed a solid media culture system for Capsella bursa-pastoris proembryos consisting of two concentric rings. The plate contains a central medium suitable to grow very immature embryos and a different peripheral medium more appropriate for the requirements of embryos at a later stage of development.
Elaborate ER methods have also been successfully applied to monocot species. In Zea mays, a double-layered medium for the culture of both zygote and proembryos was exploited [78][79][78,79]. Briefly, Matthys-Rochon et al. [79] established media for 6 DAP (pre-transitional) and 7 DAP (transitional) maize embryos. Ovaries, containing endosperm and well-organized embryos, were removed and transferred into drops of liquid MS medium [72] supplemented with 0.35 M sucrose. This first stage was indispensable for cell survival. For 7 DAP proembryos, the basal medium was NBM [80] supplemented with different concentrations of sucrose or maltose (0.18 M and 0.26 M) and solidified with gelrite (0.1%, w/v). The 6 DAP proembryos were first transferred to a bilayer culture system. To support embryo growth, the bottom layer of the N6 basal medium [73][81][73,81] was supplemented with 0.09 M sucrose and solidified with 0.8% (w/v) marine Agarose. The upper layer consisted of N6 medium supplemented with 0.35 M sucrose or 0.30 M maltose, with or without cytokinins, and solidified with 0.8% (w/v) SeaPlaque Agarose. The best results for transitional embryos (7 DAP) were obtained on media supplemented with 0.25 M maltose, resulting in 86% of plants from 200 cultured embryos. For these embryos, the embryo orientation on the surface of the culture media was crucial. For pre-transitional embryos (6 DAP), the best results (58% of plants from 52 cultured embryos) were obtained on media supplemented with 0.30 M maltose and 0.03 mM of trans-zeatin riboside (ZR). This protocol allowed the production of fertile plants in approximately two months [79].
Embryo and ovule cultures have been employed in tulips to overcome crossing barriers in interspecific hybrids, such as Tulipa gesneriana × T. fosteriana, T. gesneriana × T. eichleri, and T. gesneriana × T. greigii [82] (extensively reviewed by Marasek-Ciolakowska et al. [83]). In intraspecific crosses of T. gesneriana × T. gesneriana, Custers et al. [84] investigated the optimal conditions for culturing ovules and collecting embryos from pollinated flowers from a few days to 3 weeks. Half-strength MS medium [72] supplemented with 6% sucrose was the most suitable for obtaining larger bulbils. Culturing the ovules in Petri dishes containing a thin layer (6 mm depth) of MS medium [72] resulted in a higher rate of bulbil differentiation compared to cultures in a thick medium. For bulbil formation, the continuous dark condition was superior to a 16 h photoperiod condition. The optimal growing temperature was 12–15 °C. Based on these results, the following method was established to cultivate tulip ovules. The cultures were grown at 15 °C for 12 to 15 weeks, followed by 12 weeks at 5 °C to induce germination. Subsequently, the cultures were grown to 15 °C for 12 to 18 weeks to allow seedling growth and bulbil development. Furthermore, 6 weeks of culture at 15 °C was needed for maturation. Under improved conditions, up to 90% bulbil formation was obtained. Custers et al. [84] also compared embryo and ovule culture methods and showed that ovule culture could be applicable to save small embryos (0.3–0.7 mm in length), while isolated embryo culture yielded satisfactory results with embryos measuring at least 3 mm.
Various ER techniques have been employed to cultivate young embryos, including ovary and placenta cultures. These methods have also been combined, such as ovary culture followed by embryo or ovule culture, as well as placenta culture followed by subsequent culture of the ovary, ovule, and embryo (reviewed by Krishna et al. [19]). Overall, these ER methodologies have since been extensively employed to generate many interspecific and intergeneric hybrids [12][28][29][55][85][12,28,29,55,85]. Some examples are the applications of ER in crosses within different genera or families, such as in Phaseolus [86], Solanum [87], Cucurbita [88], Brassicaceae family [63], Manihot [89], Trifolium [90][91][92][90,91,92], Vigna [93], Elaeis [94], Paracurcuma and Eucurcuma subgenera [95], Alliums [96], and many other crosses involving phylogenetically distant species.
3.3. Medium Composition and Environmental Factors Suitable for Embryo Rescue Techniques
In vitro culture of plant cells, tissues, and organs requires of a plethora of different media, and therefore, it is impossible to establish a universal culture medium and/or standard environmental conditions suitable for the cultivation of all species and, within a single species, of all genotypes. Various aspects, such as genotype/cultivar, PGRs, chemical composition of the culture medium, and physical factors, influence the development of embryos in vitro. ER, as previously reported, can be divided into several techniques. The formulation of culture media profoundly effects the growth and development of immature and mature embryos, and many formulations have been developed for different plant species, although some are commonly used. The Murashige and Skoog (MS) formulation [72] is recognized as the most used base medium. However, there are other effective media frequently employed, including those reported by White [97], “Gamborg’s B-5” by Gamborg et al. [71], Schenk and Hildebrandt (SH) [98], Nitsch and Nitsch (NN, N6) [73], and “Woody Plant Medium” by Lloyd and McCown [99]. Additionally, plant ER media can include various components and additives, which can be placed into eight categories: water, nutrient salts (micro and macronutrients), vitamins, amino acids, carbohydrates, gelling agents, PGRs, and other organic supplements.
The process of optimizing culture media and environmental conditions is complex and time-consuming, requiring expensive chemicals to set up an efficient and different protocol for each condition.
Given these challenges, applications of artificial neural networks to in vitro plant culture systems could offer valuable insight into the prospects and potential of network technology [100]. These neural networks could be applied to various aspects of in vitro culture, including the composition of the most suitable medium for each proposed culture situation. However, the use of computer-based tools in this context is still limited, despite preliminary work demonstrating the advantages of employing these tools for analyzing large datasets [101][102][101,102]. For example, in a study by Hameg et al. [103], an experiment was optimized using three machine learning algorithms: artificial neural networks, fuzzy logic, and genetic algorithms. The objective was to unravel the essential minerals and predict the optimal combination of salts for successful in vitro micropropagation of hardy kiwi (Actinidia arguta). The authors demonstrated the suitability of computer-based tools for improving plant in vitro micropropagation of A. arguta, predicting a new mineral media formulation that improves growth response and avoids morph-physiological abnormalities. However, this new approach has proven to be valid on a case-by-case basis. Therefore, it should be considered whenever researchers are faced with different ER situations. In an overall assessment, no chemical/physiological or environmental generalizations can be made about ER, as it remains one of the most complex phases of in vitro culture of immature embryos. Nonetheless, valuable insights can be gained from the excellent reviews [12][19][26][27][28][53][12,19,26,27,28,53] regarding the optimal culture conditions to be used in different species for ER.
