Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Caglar Ersanli and Version 2 by Rita Xu.
Wounded skin can naturally be repaired by a mechanism called wound healing. Human skin is a habitat of various pathogenic and commensal bacteria. While these bacteria are in balance in healthy skin, they can lose the balance by wounding, which leads to delay in the wound-healing process. Moreover, commensal and pathogenic bacteria inhabit skin tissue and have constant communication with the immune system, which can increase and decrease the healing efficiency, respectively.
- wound healing
- skin microbiota
- manipulation strategies
1. Introduction
Cutaneous wound healing is a complicated and well-organized natural repair process that comprises four stages: hemostasis, inflammation, proliferation, and remodeling [1]. Skin damage, inducing a wound, allows organisms from foreign bodies to penetrate through the wound site [2][3]. In other words, a wound procures an occasion for both commensal and pathogenic microorganisms to access underlying tissue, then grow and colonize after reaching ideal conditions [4], which may cause further impairment in wound healing.
The complex and rich ecosystem of skin microbiota (microbiome) arises from diverse microorganisms, i.e., bacteria, fungi, viruses, and yeasts [5], and has a significant role in the protection of skin tissue and ensuring hemostasis [6][7]. The bacteria inhabiting the cutaneous microbiota can be classified as commensal and pathogenic. Pathogenic bacteria are a harmful bacteria type that can directly be transmitted to the host tissue and lead to infection. In contrast, commensal bacteria can supply essential nutrients to the host tissue and benefit in fighting infection. Although bacteria are the most abundant microorganisms in skin microbiota, only about 25% of them can move through the deeper skin layers [8], being important players in skin physiology and disease processes [9]. Commensals present in skin microbiota have been determined as beneficial with their ability to originate immune responses thanks to their communication with cutaneous cells such as keratinocytes and fibroblasts [10][11]. These types of bacteria have advantageous effects on wound healing by providing a barrier function for the skin and combating pathogenic microorganisms [12]. In contrast, pathogenic bacteria may give rise to delayed or impaired wound healing [13][14] by leading to infection in the wound site.
The crosstalk between the cutaneous microbiome, immune system, and epithelial cells is evaluated significantly for tissue repair and regeneration in vertebrates [15]. The harmony among all these provides an efficacious approach to wound healing and an invasive system by possible pathogens in equilibrium [16]. However, in the case of the disequilibrium between commensals and pathogens, cutaneous diseases may appear. In this perspective, understanding of the communication between the microbiome and the immune system as well as the identification and manipulation of the microbiome by several approaches has become prominent as an alternative solution for cutaneous wound treatment.
2. The Abundant Bacteria Implicated in Wound
Both aerobic and anaerobic microorganisms that constitute skin microbiota inhabit the skin surface soon after birth in a dynamic correlation with the host [17]. Although the bacteria protect the skin balance, they may penetrate through the underlying skin tissues when their continuity is broken by intrinsic and/or extrinsic factors. Thereafter, these penetrated bacteria can lead to the formation of colonization or contamination. Contamination is the presence of potentially pathogenic microorganisms in the wound area, whilst colonization is the existence of replicating microorganisms with no damage to the wound. However, critical colonization is the threshold that may delay the healing of the wound due to the high number of bacterial counts. Local infection with critical colonization, and proliferation of microorganisms, as well as local tissue reactions, can cause generalized host reactions, thereby an invasive infection [18]. Therefore, understanding the species and effects of the bacteria in skin tissue is important to develop solutions for infected wounds. Even though many studies have revealed the positive effect of the skin microbiota in wound healing either by modulating immune response or preventing pathogen invasion, the precise relationship between commensal microbiota and impaired wound healing remains unclear [19]. Some studies state that regardless of the destination between friend and foe, the skin microbiota tends to play a negative role in wound healing in different ways such as the elevation of pro-inflammatory mediators [20]. For instance, the persistence of bacteria in wounds impairs the healing process by elevation of pro-inflammatory cytokines such as interleukin-1 and tumor necrosis factor-alpha that in turn cause increased levels of matrix metalloproteinases (MMPs), a decreased level of tissue inhibitors to the MMPs, and decreased production of growth factors [21]. Cytokines are important signaling proteins that modulate fundamental pathophysiological and hemostatic processes, e.g., wound healing, by inducing downstream signal transduction pathways via specific cytokine receptors [22][23]. Therefore, cytokines can regulate the function of other receptors such as sodium channels, as well as the transient vanilloid receptors, and modulate the hemostatic process. Hence, a significant reduction in the number of pathogenic microbes using an appropriate antimicrobial agent is vital in regularizing wound healing. Moreover, satisfactory wound repair is possible only when the infection is brought under control. In other words, acceleration in the wound healing process is proportional to the reduction of the number of pathogenic microbes in the wound bed [24][25][26][27]. Numerous studies have reported that the most common pathogens associated with wound infections are Staphylococcus aureus (S. aureus); Pseudomonas aeruginosa (P. aeruginosa); Escherichia coli (E. coli); coagulase-negative Staphylococci (CoNS), i.e., Staphylococcus epidermidis (S. epidermidis); Streptococcus pyogenes (S. pyogenes); Klebsiella spp.; and Proteus spp. [28][29][30][31][32]. In chronic wounds, S. aureus, followed by P. aeruginosa, is the most common isolated microorganism [2][33][34], inhibiting wound healing, and is considered dominant [35][36]. However, many other commensal species have been isolated from cutaneous wounds, such as lactobacilli, which might have a positive therapeutic effect. The effect of CoNS, S. aureus, P. aeruginosa, and Lactobacilli on the wound-healing process, as illustrated in Figure 1.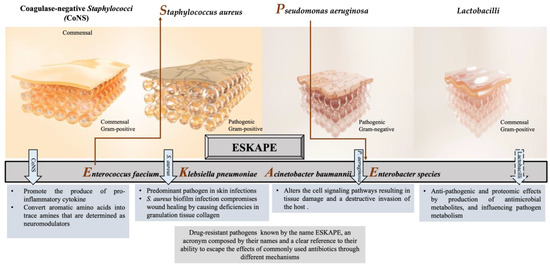
Figure 1. Different responses of the most often isolated bacteria to the process of wound healing.
2.1. Coagulase-Negative Staphylococci (CoNS)
Staphylococci (mainly S. epidermidis, S. haemolyticus, S. hominis) are generally abundant bacteria that inhabit normal skin flora. Some CoNS species demonstrate a promoting influence on the wound as well as preventing a chronic process. For example, commensals of the human skin microbiota, such as S. epidermidis, stimulate IL-17+ CD8+ T cells, which are able to produce the pro-inflammatory cytokine IL-17A and restrict pathogen intrusion [37]. Moreover, S. caprae inhibits the quorum sensing (QS) of S. aureus by forming an autoinducing peptide, which leads to the expression of diverse virulence genes [38]. On the other hand, the lantibiotics gallidermin and epidermin, which are produced from certain CoNSs or antimicrobial peptides, displayed the blockage of cell wall biosynthesis in Gram-positive bacteria that might cause the inhibition of S. aureus [39]. S. epidermidis has been getting attention with its triggering mechanisms that reduce harmful microbes and encourage wound healing in the acute phase among the other CoNSs [40]. Recent studies have shown that S. epidermidis plays an active role in skin immunity, protecting from the invasion of Gram-positive and Gram-negative pathogens [41]. The SadA-expressing S. epidermidis strains that commonly inhabit human skin and gut microbiota [31][36] can convert aromatic amino acids into trace amines that are determined as neuromodulators by interacting with diverse adrenergic receptors. The wound-healing process is enhanced thanks to this biological reaction that induces the increase in keratinocyte migration, re-epithelialization rate, and extracellular-signal-regulated kinase level [42][43]. On the other hand, trace-amine-producing skin commensals can contribute to the acceleration of wound healing by suppressing the adrenaline and represent a promising therapeutic option [44].2.2. Staphylococcus aureus
S. aureus is one of the most common and predominant pathogens with approximately 65% prevalence, involved in skin infections worldwide, which can cause persistent infections in chronic wounds with adverse effects [45]. This bacterium was first described by the Scottish surgeon Alexander Ogston as an isolate from a wound, and he demonstrated its significance as a pus pathogen [46]. As a commensal or opportunistic pathogen, S. aureus has been isolated from various reservoirs with a tendency to disseminate among them, such as humans, animals, and the environment [47][48][49], revealing genetic relatedness and sometimes threatening public health [50][51]. S. aureus is a pathobiont for humans and animals, leading to the emergence of more virulence and multidrug-resistant strains. Hence, this situation makes S. aureus-caused wound infections dangerous and requires the development of new prevention models and treatment strategies [52]. Because of the invasion, this bacterium can cause a variety of diseases, ranging from minor skin and soft tissue infections such as impetigo, folliculitis, and abscesses, to life-threatening systemic infections such as sepsis, endocarditis, or toxic shock syndrome [53]. It is also worth mentioning that the biofilm formation ability of most S. aureus strains is one of the specific virulence factors enabling them to adapt to the chronic wound environment [54]. Some S. aureus-strain-infected wounds may be challenging or almost impossible to treat due to their mostly antibiotic-resistant and high virulence nature, contributing to the pathogenicity of the host [55]. This high virulence allows it to escape the immune system’s reaction and thus the antibiotic activity. For instance, the existence of a β-lactamase enzyme in S. aureus enables it to break the ring of β-lactam antibiotics, making the antibiotics inactive [56]. In particular, some strains are resistant to methicillin as well as to other antibiotics. Methicillin resistance occurs due to the acquisition of mecA or mecC genes by previously susceptible strains that are consequently called methicillin-resistant S. aureus (MRSA) [57], followed by resistance to all β-lactam antibiotics, except for the fifth-generation cephalosporins [32][58]. MRSA is responsible for nosocomial infections, which are more difficult to treat. Patients with infections caused by MRSA have a much greater mortality risk than methicillin-sensitive strains [59]. As a consequence, the emergence and the unceasing spread of multi-resistant S. aureus strains, such as methicillin or vancomycin-resistant S. aureus, complicate the treatment of Staphylococcal infections with detrimental impact on global health and the economy. Therefore, the WHO has justifiably enlisted S. aureus as one of the major health threats in the so-called “post-antibiotic era” [60]. Thus, the development of new antibiotics and alternative prophylactic or therapeutic strategies have become inevitable to combat S. aureus, as well as other five ESKAPE bacteria (Figure 1), which possess multidrug resistance and high virulence [61][62].2.3. Pseudomonas aeruginosa
P. aeruginosa is a Gram-negative bacterium included in the ESKAPE bacteria with concerns for public health, like S. aureus, and has a place in the concept of the “one health approach” [63]. It elicits prolonged hospitalization with increased morbidity and mortality rates [64] as a common opportunistic pathogen that causes several chronic, treatment-resistant infections in humans, i.e., certain skin, respiratory, and urinary tract infections [65][66]. Moreover, it has been isolated from patients with burn wounds, cystic fibrosis, acute leukemia, organ transplants, and intravenous drug addiction [67]. This bacterium penetrates wounds, holding the ability to form intact biofilms and subsequently degrading the extracellular matrix and altering the cell signaling pathways, resulting in tissue damage and a destructive invasion of the host [68][69]. P. aeruginosa has been reported as a detrimental pathogen during the past two decades, with grounds for 10 to 20% of infections in most hospitals, being determined as one of the twelve prior pathogens that pose the greatest threat to human health according to the WHO [62]. In 2017, it was estimated that 32,600 infections were caused by multidrug-resistant P. aeruginosa among hospitalized patients, and 2700 deaths occurred in the US [70]. The emergence of multidrug-resistant P. aeruginosa resulting in the persistence and non-response to clinical treatment of infectious diseases such as infected wounds has been referred to by several studies [71][72][73]. P. aeruginosa has been evaluated as resistant to diverse antibiotics, such as β-lactams, aminoglycosides, quinolones, and sulfonamides [74][75]. This resistance is derived from its excellent ability to select chromosomal mutations and acquire resistant genes, bearing multiple antimicrobial resistance mechanisms, which led P. aeruginosa to become one of the most difficult bacteria to treat [76][77]. Different mechanisms are involved in the expression of resistance of P. aeruginosa coming from innate and acquired ways. Innate resistance is related to an overexpressed efflux pump and low permeability of the outer membrane of the bacterium [78]. Acquired resistance involves the acquisition of a resistance gene or mutation in genes encoding porins, efflux pumps, penicillin-binding proteins, and chromosomal β-lactamase, all contributing to resistance to β-lactams, carbapenems, aminoglycosides, and fluoroquinolones [79]. P. aeruginosa strains have also been found resistant to aminoglycosides carrying the mexXY genes that induce the modification of aminoglycoside enzymes [80]. On the other hand, the genes crpP and qnrVC1 have been identified in clinical isolates of P. aeruginosa, which demonstrated resistance to fluoroquinolone [81]. Hence, all these concerns become P. aeruginosa-infected wounds, making developing treatment strategies vital.3. Communication between Skin Microbiota, Immune System, and Epithelial Cells
In vertebrates, regularly, all anatomical surfaces that communicate with the environment are colonized by microbes that compose the microbiome. Therefore, the study of the physiological and metabolic effects of the human microbiome on multicellular organisms regarding both health and disease concerns has become prominent. The largest organ of the human body, the skin, is home to approximately 1012 bacterial cells [82][83] that comprise the skin microbiota, thanks to its immediate interface with the environment [17]. The skin microbiome, as mentioned, comprises a diverse population of fungi, bacteria, archaea, viruses, and sometimes parasites in close interaction with vertebrate hosts [84], and it contributes to the barrier function and hemostasis of skin tissue in various ways. For instance, the secretion of protease and lipase enzymes are involved in the desquamation and lipid surface degradation processes, respectively. Likewise, free fatty acid and sebum formation take place in the pH regulation of the skin tissue [85]. The skin microbiota has significant roles, such as the production of biofilms, bacteriocins, and quorum sensing [86][87]. It protects the skin tissue against pathogenic microorganisms by competition [88][89] and leads the production of antimicrobial peptides by virtue of commensal bacteria [39][90], which are in crosstalk with the immune system continuously that may be beneficial for the healing of the wound [91]. The internal communication between skin microbiota, epithelial cells, and the immune system is a primary mechanism to combat pathogenic invasion, as well as to ensure the maintenance of the skin commensals [14], which is schematically illustrated in Figure 2. This primary interaction has been initiated by keratinocytes toward the binding of pathogen-associated molecular patterns to pattern recognition receptors, resulting in the release of antimicrobial peptides as an inhibitory agent to diverse pathogens in the infection area. Moreover, the efficacy of various impacts of different microorganisms on the immune system has been determined as an important phenomenon in wound healing. For instance, the colony formation in wound bed by S. epidermidis has led to an increase in the expression of the cytokine, interleukin 1α (IL-1α). This cytokine is responsible for contributing to skin inflammation and host defense, which directly promotes wound healing [92]. In a study, the communication among commensal organisms, pathogens, and keratinocytes was investigated using polymicrobial biofilms formed by a mixture of commensal strains (S. epidermidis and M. luteus) and pathogens (S. aureus and P. aeruginosa). The commensals demonstrated the reduction of the damage caused by pathogens on the keratinocyte monolayer via degrading biofilm thickness and forming a layer between the keratinocytes and pathogens [93].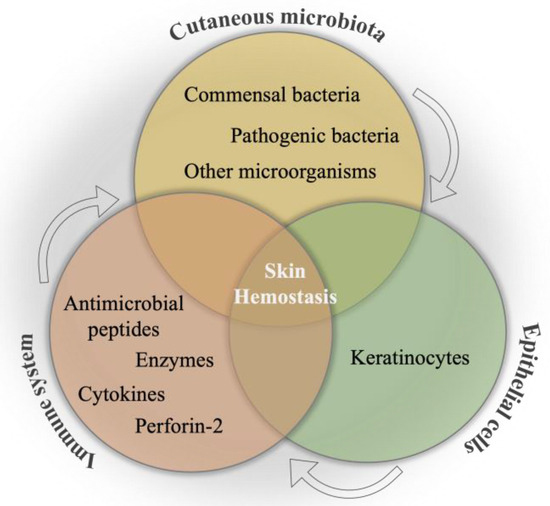
Figure 2. Schematical illustration of the relation between cutaneous microbiota, immune system, and epithelial cells for skin hemostasis.
References
- Pugliese, E.; Coentro, J.Q.; Raghunath, M.; Zeugolis, D.I. Wound Healing and Scar Wars. Adv. Drug Deliv. Rev. 2018, 129, 1–3.
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial contribution in chronicity of wounds. Microb. Ecol. 2017, 73, 710–721.
- Nasser, S.; Mabrouk, A.; Maher, A. Colonization of burn wounds in Ain Shams University burn unit. Burns 2003, 29, 229–233.
- Zeeuwen, P.L.; Boekhorst, J.; van den Bogaard, E.H.; de Koning, H.D.; van de Kerkhof, P.; Saulnier, D.M.; van Swam, I.I.; van Hijum, S.A.; Kleerebezem, M.; Schalkwijk, J. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012, 13, 1–18.
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752.
- Kelly, D.; King, T.; Aminov, R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat. Res. Fund. Mol. M. 2007, 622, 58–69.
- Macia, L.; Thorburn, A.N.; Binge, L.C.; Marino, E.; Rogers, K.E.; Maslowski, K.M.; Vieira, A.T.; Kranich, J.; Mackay, C.R. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol. Rev. 2012, 245, 164–176.
- Lange-Asschenfeldt, B.; Marenbach, D.; Lang, C.; Patzelt, A.; Ulrich, M.; Maltusch, A.; Terhorst, D.; Stockfleth, E.; Sterry, W.; Lademann, J. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Ski. Pharmacol. Physiol. 2011, 24, 305–311.
- Rosenthal, M.; Goldberg, D.; Aiello, A.; Larson, E.; Foxman, B. Skin microbiota: Microbial community structure and its potential association with health and disease. Infect. Genet. Evol. 2011, 11, 839–848.
- Pistone, D.; Meroni, G.; Panelli, S.; D’Auria, E.; Acunzo, M.; Pasala, A.R.; Zuccotti, G.V.; Bandi, C.; Drago, L. A journey on the skin microbiome: Pitfalls and opportunities. Int. J. Mol. Sci. 2021, 22, 9846.
- Harrison, O.J.; Linehan, J.L.; Shih, H.-Y.; Bouladoux, N.; Han, S.-J.; Smelkinson, M.; Sen, S.K.; Byrd, A.L.; Enamorado, M.; Yao, C. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019, 363, eaat6280.
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human microbiome: Composition and role in inflammatory skin diseases. Arch. Immunol. Ther. Exp. 2019, 67, 1–18.
- Kalan, L.R.; Meisel, J.S.; Loesche, M.A.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain-and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe 2019, 25, 641–655.
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin microbiota and its interplay with wound healing. Am. J. Clin. Dermatol. 2020, 21, 36–43.
- Wang, G.; Sweren, E.; Liu, H.; Wier, E.; Alphonse, M.P.; Chen, R.; Islam, N.; Li, A.; Xue, Y.; Chen, J. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe 2021, 29, 777–791.e776.
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The cutaneous microbiome and wounds: New molecular targets to promote wound healing. Int. J. Mol. Sci. 2018, 19, 2699.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Minasyan, H. Sepsis: Mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 1–22.
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200.
- Canesso, M.C.; Vieira, A.T.; Castro, T.B.; Schirmer, B.G.; Cisalpino, D.; Martins, F.S.; Rachid, M.A.; Nicoli, J.R.; Teixeira, M.M.; Barcelos, L.S. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J. Immunol. 2014, 193, 5171–5180.
- Tarnuzzer, R.W.; Schultz, G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325.
- Zgheib, C.; Xu, J.; Liechty, K.W. Targeting inflammatory cytokines and extracellular matrix composition to promote wound regeneration. Adv. Wound Care 2014, 3, 344–355.
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 1–9.
- Burke, J.F. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961, 50, 161–168.
- Kumar, M.S.; Sripriya, R.; Raghavan, H.V.; Sehgal, P.K. Wound healing potential of Cassia fistula on infected albino rat model. J. Surg. Res. 2006, 131, 283–289.
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170.
- Robson, M.C. Wound infection: A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. N. Am. 1997, 77, 637–650.
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96.
- Collier, M. Recognition and management of wound infections. World Wide Wounds 2004, 7, 8–14.
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174.
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52.
- Conceição, T.; Coelho, C.; Santos-Silva, I.; de Lencastre, H.; Aires-de-Sousa, M. Epidemiology of methicillin-resistant and-susceptible Staphylococcus aureus in Luanda, Angola: First description of the spread of the MRSA ST5-IVa clone in the African continent. Microb. Drug Resist. 2014, 20, 441–449.
- Guan, H.; Dong, W.; Lu, Y.; Jiang, M.; Zhang, D.; Aobuliaximu, Y.; Dong, J.; Niu, Y.; Liu, Y.; Guan, B. Distribution and antibiotic resistance patterns of pathogenic bacteria in patients with chronic cutaneous wounds in China. Front. Med. 2021, 8, 609584.
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722.
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613.
- Wong, S.Y.; Manikam, R.; Muniandy, S. Prevalence and antibiotic susceptibility of bacteria from acute and chronic wounds in Malaysian subjects. J. Infect. Dev. Ctries. 2015, 9, 936–944.
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.-J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108.
- Paharik, A.E.; Parlet, C.P.; Chung, N.; Todd, D.A.; Rodriguez, E.I.; Van Dyke, M.J.; Cech, N.B.; Horswill, A.R. Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 2017, 22, 746–756.e745.
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680.
- Claudel, J.-P.; Auffret, N.; Leccia, M.-T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A potential new player in the physiopathology of acne? Dermatology 2019, 235, 287–294.
- Stacy, A.; Belkaid, Y. Microbial guardians of skin health. Science 2019, 363, 227–228.
- Pullar, C.E.; Isseroff, R.R. The β2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J. Cell Sci. 2006, 119, 592–602.
- Steenhuis, P.; Huntley, R.; Gurenko, Z.; Yin, L.; Dale, B.; Fazel, N.; Isseroff, R. Adrenergic signaling in human oral keratinocytes and wound repair. J. Dent. Res. 2011, 90, 186–192.
- Luqman, A.; Muttaqin, M.Z.; Yulaipi, S.; Ebner, P.; Matsuo, M.; Zabel, S.; Tribelli, P.M.; Nieselt, K.; Hidayati, D.; Götz, F. Trace amines produced by skin bacteria accelerate wound healing in mice. Commun. Biol. 2020, 3, 277.
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2020, 271, 1174.
- Alexander, O. Classics in infectious diseases. On abscesses. Rev. Infect. Dis 1984, 6, 122–128.
- Heaton, C.J.; Gerbig, G.R.; Sensius, L.D.; Patel, V.; Smith, T.C. Staphylococcus aureus epidemiology in wildlife: A systematic review. Antibiotics 2020, 9, 89.
- Pirolo, M.; Visaggio, D.; Gioffrè, A.; Artuso, I.; Gherardi, M.; Pavia, G.; Samele, P.; Ciambrone, L.; Di Natale, R.; Spatari, G. Unidirectional animal-to-human transmission of methicillin-resistant Staphylococcus aureus ST398 in pig farming; evidence from a surveillance study in southern Italy. Antimicrob. Resist. Infect. Control. 2019, 8, 1–10.
- Voidarou, C.; Tzora, A.; Skoufos, I.; Vassos, D.; Galogiannis, G.; Alexopoulos, A.; Bezirtzoglou, E. Experimental effect of ozone upon some indicator bacteria for preservation of an ecologically protected watery system. Water Air Soil Pollut. 2007, 181, 161–171.
- Patrick, S. Bacteroides. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 917–944.
- Salgueiro, V.; Manageiro, V.; Bandarra, N.M.; Ferreira, E.; Clemente, L.; Caniça, M. Genetic relatedness and diversity of Staphylococcus aureus from different reservoirs: Humans and animals of livestock, poultry, zoo, and aquaculture. Microorganisms 2020, 8, 1345.
- Mrochen, D.M.; Fernandes de Oliveira, L.M.; Raafat, D.; Holtfreter, S. Staphylococcus aureus host tropism and its implications for murine infection models. Int. J. Mol. Sci. 2020, 21, 7061.
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661.
- Paleczny, J.; Junka, A.; Brożyna, M.; Dydak, K.; Oleksy-Wawrzyniak, M.; Ciecholewska-Juśko, D.; Dziedzic, E.; Bartoszewicz, M. The high impact of Staphylococcus aureus biofilm culture medium on in vitro outcomes of antimicrobial activity of wound antiseptics and antibiotic. Pathogens 2021, 10, 1385.
- Alavi, S.M.; Khosravi, A.D.; Sarami, A.; Dashtebozorg, A.; Montazeri, E.A. Bacteriologic study of diabetic foot ulcer. Pak. J. Med. Sci. 2007, 23, 684.
- Fatimah, S.; Nadifah, F.; Burhanudin, I. Uji daya hambat ekstrak etanol kubis (Brassica oleracea var. capitata f. alba) terhadap bakteri Staphylococcus aureus secara in vitro. Biogenesis J. Ilm. Biol. 2016, 4, 102–106.
- Boswihi, S.S.; Udo, E.E. Methicillin-resistant Staphylococcus aureus: An update on the epidemiology, treatment options and infection control. Curr. Med. Res. Pract. 2018, 8, 18–24.
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18.
- Tacconelli, E.; Pop-Vicas, A.; D’Agata, E. Increased mortality among elderly patients with meticillin-resistant Staphylococcus aureus bacteraemia. J. Hosp. Infect. 2006, 64, 251–256.
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary; World Health Organization: Geneva, Switzerland, 2014.
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539.
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327.
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in hospitals: How understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control. 2020, 9, 1–17.
- Alhussain, F.A.; Yenugadhati, N.; Al Eidan, F.A.; Al Johani, S.; Badri, M. Risk factors, antimicrobial susceptibility pattern and patient outcomes of Pseudomonas aeruginosa infection: A matched case-control study. J. Infect. Public Health 2021, 14, 152–157.
- Raizman, R.; Little, W.; Smith, A.C. Rapid diagnosis of Pseudomonas aeruginosa in wounds with point-of-care fluorescence Imaing. Diagnostics 2021, 11, 280.
- Vanderwoude, J.; Fleming, D.; Azimi, S.; Trivedi, U.; Rumbaugh, K.P.; Diggle, S.P. The evolution of virulence in Pseudomonas aeruginosa during chronic wound infection. Proc. R. Soc. B 2020, 287, 20202272.
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313.
- Prasad, A.S.B.; Shruptha, P.; Prabhu, V.; Srujan, C.; Nayak, U.Y.; Anuradha, C.K.R.; Ramachandra, L.; Keerthana, P.; Joshi, M.B.; Murali, T.S. Pseudomonas aeruginosa virulence proteins pseudolysin and protease IV impede cutaneous wound healing. Lab. Investig. 2020, 100, 1532–1550.
- Schmidtchen, A.; Holst, E.; Tapper, H.; Björck, L. Elastase-producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microb. Pathog. 2003, 34, 47–55.
- U.S. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United State; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob. Agents Chemother. 2006, 50, 43–48.
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019, 32, e00031-19.
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s not easy being green: A narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42.
- Miyoshi-Akiyama, T.; Tada, T.; Ohmagari, N.; Viet Hung, N.; Tharavichitkul, P.; Pokhrel, B.M.; Gniadkowski, M.; Shimojima, M.; Kirikae, T. Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa. Genome Biol. Evol. 2017, 9, 3238–3245.
- Sonmezer, M.C.; Ertem, G.; Erdinc, F.S.; Kaya Kilic, E.; Tulek, N.; Adiloglu, A.; Hatipoglu, C. Evaluation of risk factors for antibiotic resistance in patients with nosocomial infections caused by Pseudomonas aeruginosa. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 1321487.
- Montero, M.M.; López Montesinos, I.; Knobel, H.; Molas, E.; Sorlí, L.; Siverio-Parés, A.; Prim, N.; Segura, C.; Duran-Jordà, X.; Grau, S. Risk factors for mortality among patients with Pseudomonas aeruginosa bloodstream infections: What is the influence of XDR phenotype on outcomes? J. Clin. Med. 2020, 9, 514.
- Poole, K.; Krebes, K.; McNally, C.; Neshat, S. Multiple antibiotic resistance in Pseudomonas aeruginosa: Evidence for involvement of an efflux operon. J. Bacteriol. Res. 1993, 175, 7363–7372.
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067.
- Oie, S.; Fukui, Y.; Yamamoto, M.; Masuda, Y.; Kamiya, A. In vitro antimicrobial effects of aztreonam, colistin, and the 3-drug combination of aztreonam, ceftazidime and amikacin on metallo-β-lactamase-producing Pseudomonas aeruginosa. BMC Infect. Dis. 2009, 9, 1–5.
- Atassi, G.; Scheetz, M.; Nozick, S.; Rhodes, N.J.; Murphy-Belcaster, M.; Murphy, K.R.; Ozer, E.A.; Hauser, A.R. Genomics of aminoglycoside resistance in pseudomonas aeruginosa bloodstream infections at a United States Academic Hospital. Medrxiv 2021, 11, e05087-22.
- Khan, M.; Summers, S.; Rice, S.A.; Stapleton, F.; Willcox, M.D.; Subedi, D. Acquired fluoroquinolone resistance genes in corneal isolates of Pseudomonas aeruginosa. Infect. Genet. Evol. 2020, 85, 104574.
- Berg, R.D. The indigenous gastrointestinal microflora. Trends Microbiol. 1996, 4, 430–435.
- Savage, D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977, 31, 107–133.
- Ross, A.A.; Rodrigues Hoffmann, A.; Neufeld, J.D. The skin microbiome of vertebrates. Microbiome 2019, 7, 1–14.
- Meisel, J.S.; Sfyroera, G.; Bartow-McKenney, C.; Gimblet, C.; Bugayev, J.; Horwinski, J.; Kim, B.; Brestoff, J.R.; Tyldsley, A.S.; Zheng, Q. Commensal microbiota modulate gene expression in the skin. Microbiome 2018, 6, 1–15.
- Baldwin, H.E.; Bhatia, N.; Friedman, A.; Prunty, T.; Martin, R.; Seite, S. The role of cutaneous microbiota harmony in maintaining a functional skin barrier. Skin 2017, 1, s139.
- Williams, M.R.; Costa, S.K.; Zaramela, L.S.; Khalil, S.; Todd, D.A.; Winter, H.L.; Sanford, J.A.; O’Neill, A.M.; Liggins, M.C.; Nakatsuji, T. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 2019, 11, eaat8329.
- Grice, E.A.; Kong, H.H.; Renaud, G.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Wolfsberg, T.G.; Turner, M.L.; Segre, J.A. A diversity profile of the human skin microbiota. Genome Res. 2008, 18, 1043–1050.
- Kong, H.H.; Andersson, B.; Clavel, T.; Common, J.E.; Jackson, S.A.; Olson, N.D.; Segre, J.A.; Traidl-Hoffmann, C. Performing skin microbiome research: A method to the madness. J. Investig. Dermatol. 2017, 137, 561–568.
- O’Sullivan, J.N.; Rea, M.C.; O’Connor, P.M.; Hill, C.; Ross, R.P. Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiol. Ecol. 2019, 95, fiy241.
- Coates, M.; Lee, M.J.; Norton, D.; MacLeod, A.S. The skin and intestinal microbiota and their specific innate immune systems. Front. Immunol. 2019, 10, 2950.
- Maheswary, T.; Nurul, A.A.; Fauzi, M.B. The insights of microbes’ roles in wound healing: A comprehensive review. Pharmaceutics 2021, 13, 981.
- Jordana-Lluch, E.; Garcia, V.; Kingdon, A.D.; Singh, N.; Alexander, C.; Williams, P.; Hardie, K.R. A simple polymicrobial biofilm keratinocyte colonization model for exploring interactions between commensals, pathogens and antimicrobials. Front. Microbiol. 2020, 11, 291.
More
