Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by George Kordas and Version 2 by Lindsay Dong.
Countries that do not have oil and natural gas but are forced to reduce pollution due to combustion have stimulated and developed new technologies for absorption, storage, and energy creation based on nanotechnology. These new technologies are up-and-coming because they will solve the problem without additional environmental burden.
- nanotechnology
- nanocontainers
- paraffin
- thermal energy storage
- household equipment
- light microtraps
- nanoparticle energy conversion
- light absorption
- electricity generation
1. Introduction
The rehabilitation of oil and natural gas in countries that do not have such energy resources and the reduction in pollution due to combustion has led to the research and development of new technologies for the absorption and storage of solar energy [1]. We know that energy is not created or destroyed but preserved [2]. This principle is the basis of the technology of storing and exploiting solar energy from a period of low demand to a period of high demand. Renewable energy sources and storage technologies offer solutions to replace some fuels and make them life-saving solutions for the future. Energy sources, such as wind, solar, etc., will only apply if ways of storing the produced energy are found simultaneously. The generated energy can be stored with phase change materials (PCMs) [3][4][5][3,4,5]. The application of PCMs is essential where electricity is expensive or in areas where the electricity supply could be more practical.
PCMs change phases at a specific temperature achieved through internal energy transfer as a heat transfer that we call “latent heat”. Liquid-dissolved PCMs create another category of materials we call phase change slurries (PCSs), enclosed in heat transfer circuits [6]. PCS can contain up to 40% PCMs with a melting temperature of 60 °C and have a lower physical coefficient of thermal permeability compared to water. One application is water heaters, which in recent times are now on the market under operating conditions. Recent review publications present such applications [7][8][7,8]. This publication examines phase change memory (PCM) materials encapsulated into containers to improve the performance of devices. Encapsulating PCMs in nanocontainers is a one-way solution for many applications. PCMs on a paraffin basis dissolve on objects that are applied, e.g., electronic devices, thereby corroding these devices. Their isolation in a container is indicated to isolate paraffin from the environment and use its function as an energy storage/cooling material. Another application concerns the slurries where the systems need to be protected from the solution environment to act for a long time as a kind of heat capacity enhancement of this solution. These shells guarantee the long-term operation of the PCM materials. Another severe problem solved by encapsulating the PCMs into containers is the reduction in temperature gradients during heat transfer inside or outside the material during heat transfer in and out of the material. In the last few years, many works have concerned the development of silica shells [1], styrene-methyl methacrylate copolymer [2][3][2,3], urea-formaldehyde (UF) [4][5][4,5], polystyrene (PS) [6], melamine formaldehyde (MF) [7], polymethyl methacrylate (PMMA) [8][9][10][11][8,9,10,11], polycarbonate (PC) [3][12][3,12], and polyurethane (PU) [13][14][15][13,14,15].
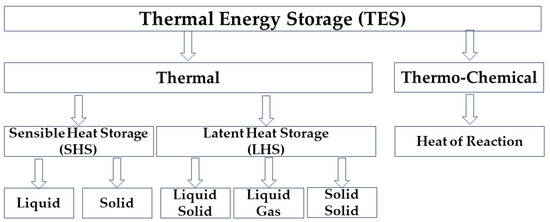
2. Thermal Energy Storage (TES)
Thermal energy storage is achieved through internal energy change, such as thermal (sensible heat and latent heat) and thermo-chemical [10]. Figure 12 summarizes these methods, but in the rest of the text, there will be a detailed description of the possibilities of storing thermal energy through latent heat.
Figure 12.
Forms of energy storage [11].
2.1. Sensible Heat Storage (SHS)
Sensible heat storage (SHS) materials do not change during heat storage [12]. These materials can be liquids or solids. Energy storage, Q, is carried out by changing the temperature of the material via a charging and discharging process as well as depends on the thermal specific heat capacity, Cp, and the mass, m, of the material:
Q = m Cp (Tf − Ti)
The parameters T
i
and Tf
are the initial and final temperatures, respectively.
SHS systems must have a high specific heat capacity but at the same time be stable at the time of their application, be compatible with the environment of use, have a high density, be cheap, and have almost zero CO2
residues. A space problem often requires as high energy density as possible. Still, the manufacturer must ensure good thermal insulation of the SHS storage area, especially when the periods of charge and discharge are long, to limit heat losses.
2.2. Latent Heat Storage (LHS)
When the phase change achieves energy absorption and release, you are subjected to (Solid ↔ Solid, Solid ↔ Liquid, and Liquid ↔ Gas) material; the entry is talking about latent heat storage based on phase change from one physical state to another [14][15]. When a Solid ↔ Solid transition is achieved, the material is converted from one crystalline form to another, where the volume changes are relatively small compared to the volume changes one observes in Sold ↔ Liquid and Liquid ↔ Gas transitions. Pentaglycerine is a Solid ↔ Solid PCM with promising properties such as heat of fusion (16 kJ/kg) and phase transition point (81 °C) [1][16]. Solid ↔ Gas transitions may have higher latency heat values. However, it is not easy for the gas created during Solid ↔ Gas transformation to coexist, which makes these PCMs unsuitable for many applications. On the contrary, Solid ↔ Liquid PCMs are economically attractive. They are used in latent heat storage systems that aim to reduce the fluctuation of the interior temperature due to fluctuating solar radiation [17].
When the phase change achieves energy absorption and release, you are subjected to (Solid ↔ Solid, Solid ↔ Liquid, and Liquid ↔ Gas) material; the entry is talking about latent heat storage based on phase change from one physical state to another [14,15]. When a Solid ↔ Solid transition is achieved, the material is converted from one crystalline form to another, where the volume changes are relatively small compared to the volume changes one observes in Sold ↔ Liquid and Liquid ↔ Gas transitions. Pentaglycerine is a Solid ↔ Solid PCM with promising properties such as heat of fusion (16 kJ/kg) and phase transition point (81 °C) [1,16]. Solid ↔ Gas transitions may have higher latency heat values. However, it is not easy for the gas created during Solid ↔ Gas transformation to coexist, which makes these PCMs unsuitable for many applications. On the contrary, Solid ↔ Liquid PCMs are economically attractive. They are used in latent heat storage systems that aim to reduce the fluctuation of the interior temperature due to fluctuating solar radiation [17].
Summing up, heat storage in these materials is based on capturing or releasing heat through a PCM phase change. Energy storage, Q, is given by the following formula:
Q = m [Cs
, (Tm
− T) + am
Δhm
+ Cp
(Tf
− Tm
)]
where m is the mass of the melted PCM, Δhm
, Csp
, and Clp
are the specific heat of the solid and liquid PCM at temperatures Tm
− Tl,
and Th
− Tm
, respectively; this equation expresses the greater Δh, the extended cooling effect.
The imprint from this equation is that LHS achieves maximum energy density per unit of mass and volume. LHS systems suitable for space cooling have Tm
between 10 and 30 °C, and space heating systems have Tm
between 30 and 100 °C.
2.3. Paraffin
Paraffin consists of a chain CH3
-(CH2
)-CH3
that the segment (CH3
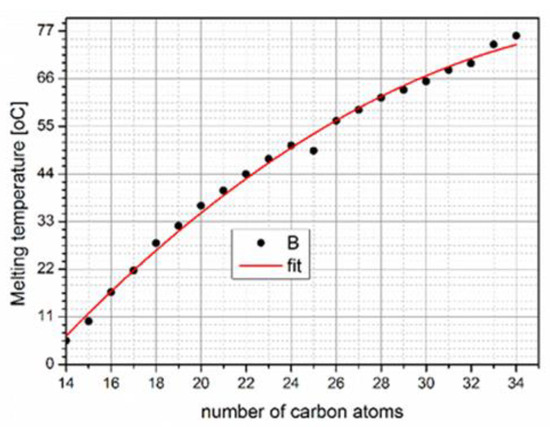
) crystallizes, releasing a sizeable latent heat. The melting point and latent heat fusion depend on the chain length (Figure 2). With the increase in the melting temperature, as the carbon number increases (
3). With the increase in the melting temperature, as the carbon number increases (Figure 2), heat storage capacity also increases. Various works in the literature have demonstrated that paraffin has good thermal stability and stable properties after 1000–2000 cycles [18]. Furthermore, paraffin possesses high latent heat, thermal stability, chemical stability, and sufficient mechanical strength while also being non-toxic, non-corrosive, and non-supercooling. On the other hand, leakage can create problems [19][20]. Thus, the manufacture of composite PCM after encapsulating it in a highly conductive material can reduce the pain and, at the same time, affect the ability to store heat [21]. The ol-gel method is one widely used manufacturing method adopted by various researchers for preparing paraffin PCM composites [22][23].
3), heat storage capacity also increases. Various works in the literature have demonstrated that paraffin has good thermal stability and stable properties after 1000–2000 cycles [18]. Furthermore, paraffin possesses high latent heat, thermal stability, chemical stability, and sufficient mechanical strength while also being non-toxic, non-corrosive, and non-supercooling. On the other hand, leakage can create problems [19,20]. Thus, the manufacture of composite PCM after encapsulating it in a highly conductive material can reduce the pain and, at the same time, affect the ability to store heat [21]. The ol-gel method is one widely used manufacturing method adopted by various researchers for preparing paraffin PCM composites [22,23].

Lately, there has been an increasing number of publications concerning paraffin in containers. These publications are concerned with awareness of problems related to conductivity and leakage, stability of the shells in solutions at higher temperatures, and the other disadvantages of encapsulation. These problems can be solved by combining them with metallic or non-metallic particles, fibrous materials, expanded or porous materials, and additives that slow flame spread.
Non-paraffin is a phase change material that provides energy storage [24][40]. It can be esters, fatty acids, alcohols, and glycols. Some of the characteristics of these organic materials are as follows: (i) high heat of melting, (ii) some are flammable, (iii) low thermal conductivity, (iv) low flash points, (v) different levels of toxicity, and (vi) instability at high temperatures. Saturated fatty acids have the formula CH3(CH2)2nCOOH [25][41]. Fatty acids have a reproducible melting and freezing point, showing minimal phase transition volume changes [26][42]. Their disadvantages are low thermal conductivity and cost, which is higher than paraffin [25][41]. Fatty acid esters are produced from acids where one alkyl group replaces a hydroxyl group. The transition temperature is narrow, and their mixtures form eutectics with negligible subcooling. These are available in the cosmetics and clothing industries and are widely available [27][43]. Sugar alcohol has the usual expression HOCH2(CHOH)nCH2OH. They have latent fusion heat of about 300 kJ/kg and 375 MJ/m3. These have a high melting temperature of over 90 °C and can be flammable, which makes them difficult to use in buildings [28][44]. Polyethylene glycols (PEGs) have the chemical formula H.O.–CH2–(CH2–O–CH2–)n–CH2–O.H. They exhibit chemical and thermal stability and are non-flammable, non-toxic, non-corrosive, and cheap. Their thermal conductivity is low. These PCMs’ melting point and latent fusion heat increase as their molecular weight increases [29][45].2.4. Non-Paraffins
2.5. Inorganic Phase Change Materials
Inorganic materials are subordinated to salt hydrates and minerals [24][28][30][31][32][33][34][35][36][37][40,44,50,51,52,53,54,55,56,57]. These phase-change materials do not thaw significantly, and the melting heat does not change with repeated transitions. Salt hydrates contain a crystalline solid of the general formula AB-nH2O. The solid–liquid transformation of salt hydrates is saltwater dehydration, although this process resembles thermodynamic melting or cooling. Because they have a high density, the latent heat of fusion per volume is greater than that of organic materials [12]. The thermal conductivity is low to moderate but flammable and cheaper than paraffin. However, they are thermally unreliable for long periods of operation because the phase separation occurs under cooling and heating cycles [38][58]. One way to avoid this problem is to add fattening agents. The melting process leading to salt and water causes salt precipitation due to the difference in density between salt and water and changing the stoichiometry after several cycles. Other materials, such as clay, must be added to extend their lifespan and eventually reach 1000 cycles without altering their properties [39][59]. Mg(NO3)2·6H2O were incorporated in nano-poly(ethyl-2-cyanoacrylate) capsules with 100 to 200 nm size. The DSC method showed the stability of the salt hydrates, which remained unchanged after 100 thermal cycles, with a latent heat of 83.2 J/g. This method of encapsulation and volume improvements of Mg(NO3)2·6H2O in nanocontainers guides its structural integrity and chemical stability. Using the sol-gel process, the sodium thiosulfate pentahydrate (SoTP) was encapsulated in silica containers. According to SEM measurements, the MicroPCM has a spherical shape of approximately 200 nm. Supercooling has significantly been reduced. The thermal conductivity of SoTP in silica varied between 0.6035 and 0.7718 W/(m·k) [40][61]. Na2SO4 10H2O suffers from phase separation and supercooling in heat storage applications. These effects can be diminished when implanted in silicon nanocontainers. Its inclusion of Na2SO4 10H2O@SiO2 from the SiO2 matrix suspends its supercooling due to its limitation to the nanocontainer. Microcapsules with modified methyl polymethacrylate (PMMA) were loaded with disodium hydrogen phosphate heptahydrate (Na2HPO4-7H2O), and their thermal properties were tested. The analysis showed an improved degree of supercooling, making this a valuable combination for thermal energy storage materials [41][63]. A eutectic mixture is one compound consisting of two or more components, such as mixtures of organic–organic, organic–inorganic, and inorganic–inorganic compounds. The eutectic compound changes phases without phase separation and has a single cooling–melting point [26][42]. Note that their melting temperature is lower than their compounds [12].2.6. Phase Change Materials (PCMs)
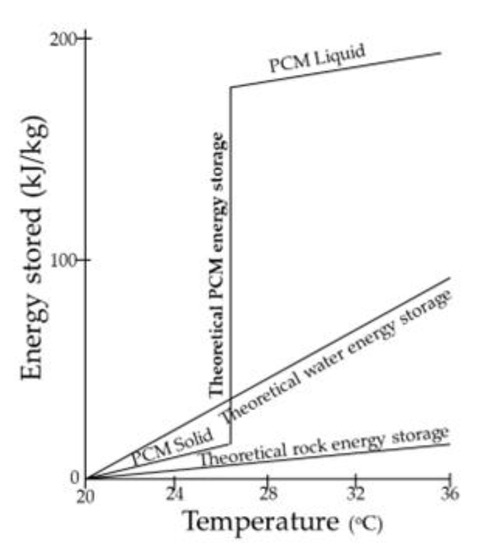
Heat storage in materials based on phase change is called latent heat storage and occurs when a material is converted from solid to liquid or liquid to solid [4]. The temperature in these materials rises as they absorb heat. PCMs absorb and give off heat almost constantly (Figure 36). As a result, they store 5–14 times more heat per unit volume than logical storage materials such as water, masonry, or rock. These materials must exhibit specific desirable thermodynamic, kinetic, and chemical properties. In addition, there are economic criteria for their use. One important consideration is the easy availability of these materials.
Figure 36.
PCM energy storage.
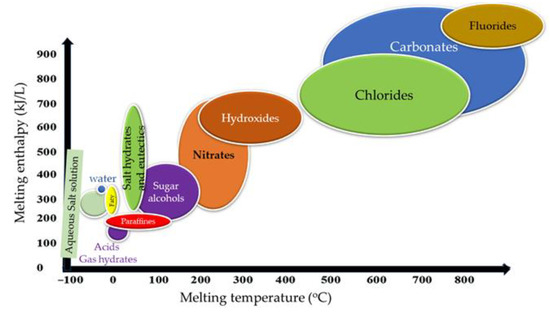
Figure 47 shows the fusion heat versus the melting temperature of the various PCMs. This figure shows the multiple PCMs’ defined temperature and enthalpy ranges that determine their applications. Paraffin, fatty acids, and hydrates are suitable for producing low-temperature heat (up to 100 °C) for use in the building and solar thermal industries, thermal storage in structural elements, and ventilation and air conditioning thermos units. With comprehensive service in the range of medium temperatures from 100 to 250 °C, this area can intersect the needs of the manufacturing sector and the whole range of heating and cooling needs of large industries. Many industries in this area also generate small-scale electricity, including in tourist resorts’ cooling and heating facilities and devices operating in this area, such as solar cookers, from where these PCMs are necessary. The encapsulation of PCMs in containers of various sizes, from a few microns to a few centimeters, is required in many applications where they meet the devices. It can lead to corrosion or dispersion that follows their dissolution and deactivation [7].
 Many PCMs have the “supercooling” effect, resulting in the crystallization rate being very low in temperature compared to the melting point. Supercooling is a state where the liquid solidifies below the melting point, and the lag in the melting process only depends on the discharge speed from this material starting from the melting temperature.
Many PCMs have the “supercooling” effect, resulting in the crystallization rate being very low in temperature compared to the melting point. Supercooling is a state where the liquid solidifies below the melting point, and the lag in the melting process only depends on the discharge speed from this material starting from the melting temperature.
The sample and materials are heated constantly while DSC or DTA curves and ΔT are recorded between the sample and the reference material. The reference material recommended is alumina. The latent heat of melting is calculated using the area below the top of the melting curve and calculated from the tangent at the curve’s base. Recent literature describes in detail how these measurements are made, as well as the analyses of these measurements [42][70].
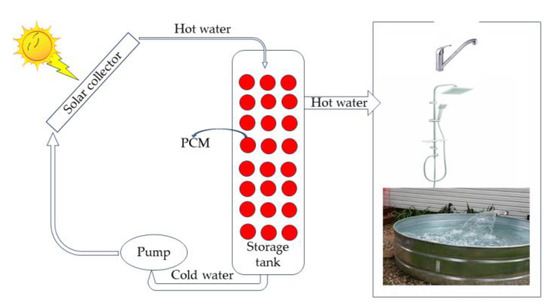

Figure 47.
Melting enthalpy vs. melting temperature for most common PCMs.
2.7. Classification
Table 16 suggests that the PCMs are classified as organic, inorganic, and eutectic. Table 16 shows these categories of materials. Organic PCMs are divided into paraffin and non-paraffin PCMs. Inorganic PCMs include hydrates, metals, and alloys. These have high latent heat and thermal conductivity and are non-toxic and non-flammable, contrasting with the organic PCMs.Table 16.
Classification of PCMs.
| PCM | ||
|---|---|---|
| ↓ | ||
2.8. Techniques for Measuring the Latency of Melting Heat and Melting Temperature
The methods used to estimate the latent heat of melting and melting temperature of PCMs are as follows:-
Differential thermal analysis (DTA);
-
Differential scanning calorimeter (DSC).
| ↓ | ↓ | ↓ |
| Organic | Inorganic | Eutectic |
| ↓ | ↓ | |
| ParaffinNon-Paraffin | Salt-HydratesMetal | Organic-Organic |
| Organic-Inorganic | ||
| Inorganic-Inorganic |
3. Solar Water Heating Systems
A solar water heater is a case where PCMs can be applied since they are relatively cheap materials and do not raise costs but only increase the efficiency of the water heater and insulation compared to polyurethane. During the hours of sunshine, the water heats until the heat is transferred to the PCM when the temperature reaches the material’s melting point. The PCM gathers energy in the form of latent heat and melts. During long hours of sunshine, hot water is withdrawn and replaced by cold water, which gains power from PCM. The PCM’s energy in its phases changes from liquid to solid. Therefore, constructing a water heater unit with PCM will be integrated into its thermal insulation system. The hot water can be transferred from the water heaters to a heat storage system located in the basement of a house that will return to where it is needed in sunless hours. An isolated sizeable cylindrical storage tank was used to experimentally investigate the material storage performance of paraffin wax in small spherical aluminum containers. The aluminum containers are commercially available. It was found that a PCM-loaded storage device has an advantage of 13 to 10 4 °C in hot water storage compared to a layout without PCMs. During a 24 h trial, the stored water temperature remained 30 °C higher than the ambient temperature. One can use such devices for one’s everyday routine [43][71]. Emphasis has been placed in the literature on improving the efficiency of commercial solar water systems by using PCM throughout the heating season [44][73]. PCM particles from 1 to 3 mm have been used in thermal energy storage, and analysis showed that the application was beneficial inside a water tank with a significant drop-in heat transfer rate [45][46][74,75]. Such a system is believed to be more flexible and responsive for energy storage at a reduced volume [45][74]. It was found that the temperature difference required to transfer the heat coefficient by natural convection is reduced by quite a large percentage [44][73]. Solar water heating in a solar collector system can significantly improve efficiency if the water tank is structured with phase change materials. One experiment used 2.5 kg of paraffin wax cut into a cylindrical aluminum casing with a total % PCM volume of 3%. The outer diameters of the cylindrical geometry were at a height of 0.3 m, and the inner diameter was 0.127 m. The temperature distribution along the size of the storage tank the energy and energy efficiency of the charge and the collector's efficiency were finally calculated and compared with CFD software. The system’s energy efficiency improved by up to 36.48% due to improved heat transfer between PCM and water [47][76]. Figure 58 shows a typical heat transfer unit consisting of a large, medium container, into which spheres of a few centimeters containing paraffin are incorporated and organized in a class. Among these spheres is a slurry consisting of nanospheres, which may be a kind of paraffin, encapsulated silica spheres, which flow undisturbed into the large spheres and exit the end of this arrangement. The thermal properties of such an installation depend on the type of pools and fluid used, the size of the facility, i.e., cross-sectional area and length, the slurry flow rate, and the temperature of the slurry inlet [48][81]. The device stores thermal energy during the day with sunshine and returns to the heating and cooling system of buildings on demand, where a second circuit is activated when the ambient temperature drops to the point that requires the commissioning of this device [7][43][7,71]. Depending on the thermal energy storage system, one can use these facilities for heating swimming pools, buildings, and other household appliances, depending on the system’s architectural design.
Figure 58. Solar collectors can heat the water of a water heater and a hot water storage system in the basement of a house, which can use during periods when sunshine is low [12][49][50][51][12,82,83,84].
4. Solar Cookers
Solar cookers are one of the significant applications of PCMs that can be used for cooking when there is no sun (Figure 69). The kitchen will use PCM with latent heat and storage materials in a solar cooker-type frame to cook food late at night. Solar cookers are limited to lots of sunshine and equipped with heat storage systems above 100 °C. PCMs can be magnesium nitrate hexahydrate (Mg(NO3)2-6H2O) as a PCM for heat storage [52][85].
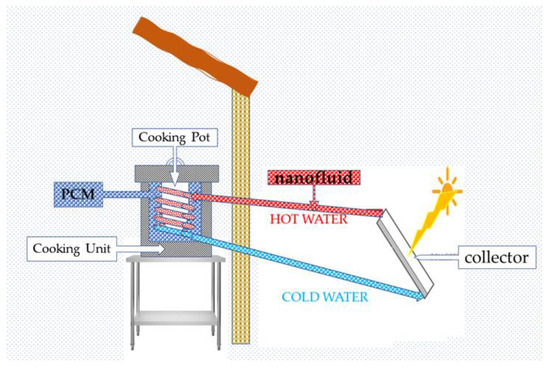

Figure 69.
Solar cookers.
Solar cookers are feasible in countries of plentiful sunshine under three conditions: the solar concentrate, which must be a pipe or parabolic arrangement, and the appropriate backing stove with good insulation, as shown in Figure 69. Appropriate nanofluids and PCMs must also be investigated for reducing cooking time and optimizing the temperature the device can achieve.
5. Solar Green House
In greenhouses and closed-end fish farms, PCM will store solar energy for the heating and drying process and plant, spirulina, and shrimp production. PCM-type CaC12-6H2O in the aerosol cans will store energy inside and outside the facility. In addition, buildings will use these facilities for air conditioning (Figure 710) [24][38][51][53][54][55][56][57][58][40,46,58,84,91,92,93,94,95].
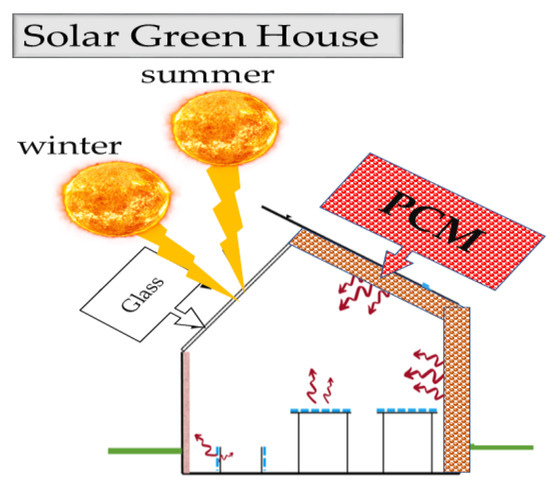

Figure 710. Solar greenhouse.
6. Buildings
PCMs have been developed for thermal storage in buildings. PCM is integrated into walls, plasterboard, shutters, insulation, and underfloor heating systems as part of the building for heating and cooling applications [53][58][59][60]. The purpose is to shift the peak load and harness solar energy. The implementation of PCMs in the building can have two different objectives. They first used natural heat as solar energy for heating or cooling. Second, they used artificial heat or complex sources. In any case, storage of heat or cold is necessary to match availability and demand over time. PCMs will be used in wall construction, building elements other than the walls, storage plants, plasterboard, bricks, and paints (
Solar greenhouse.
6. Buildings
PCMs have been developed for thermal storage in buildings. PCM is integrated into walls, plasterboard, shutters, insulation, and underfloor heating systems as part of the building for heating and cooling applications [46,95,97,98]. The purpose is to shift the peak load and harness solar energy. The implementation of PCMs in the building can have two different objectives. They first used natural heat as solar energy for heating or cooling. Second, they used artificial heat or complex sources. In any case, storage of heat or cold is necessary to match availability and demand over time. PCMs will be used in wall construction, building elements other than the walls, storage plants, plasterboard, bricks, and paints (
Figure 8).
11).
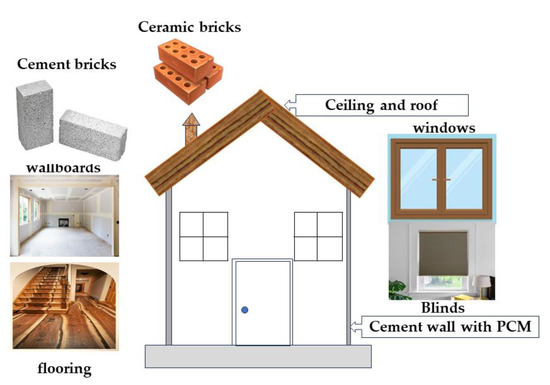

Figure 811. PCMs in buildings.
7. Textiles
An essential application in the future can be in fabrics that contain phase change materials and immediately mitigate external temperature changes and maintain a constant body temperature [61]. Encapsulating PCM in capsules and incorporating these into textile products provides tremendous energy storage benefits and protects the body from temperature changes. These can be used to irrigate bed linen, and in various clothing technologies, constant temperatures are necessary [61]. The PCM capsules are planned to be incorporated into acrylic fiber yarns that, with some processing, will be subjected to standard textile machinery. When the outside temperature rises, the microcapsules absorb heat and store it as liquefaction of phase change materials. The opposite happens when the temperature drops; worse phase change materials solidify again, releasing temperature. These possibilities can be realized with capsules containing PCMs incorporated into garments. The microcapsules must be incorporated into the fibers of the fabrics directly to be woven into the final product. Gelatin/sodium alginate/clay capsules encapsulated n-eicosane and examined whether they can improve fabrics’ thermal comfort and flame-retardant properties.
An essential application in the future can be in fabrics that contain phase change materials and immediately mitigate external temperature changes and maintain a constant body temperature [101]. Encapsulating PCM in capsules and incorporating these into textile products provides tremendous energy storage benefits and protects the body from temperature changes. These can be used to irrigate bed linen, and in various clothing technologies, constant temperatures are necessary [101]. The PCM capsules are planned to be incorporated into acrylic fiber yarns that, with some processing, will be subjected to standard textile machinery. When the outside temperature rises, the microcapsules absorb heat and store it as liquefaction of phase change materials. The opposite happens when the temperature drops; worse phase change materials solidify again, releasing temperature. These possibilities can be realized with capsules containing PCMs incorporated into garments. The microcapsules must be incorporated into the fibers of the fabrics directly to be woven into the final product. Gelatin/sodium alginate/clay capsules encapsulated n-eicosane and examined whether they can improve fabrics’ thermal comfort and flame-retardant properties.
8. Nano- and Micro-Encapsulation of PCM
Caprylic acid is a PCM that can find use in sectors such as construction, textiles, and agriculture because it has a melting point of about 16 °C and a latent heat storage capacity of 158 J/g. It is in liquid form at room temperature, and under such conditions, it is not easy to use. Encapsulating caprylic acid in capsules is the one-way solution for its use as a PCM [62][49]. This is why the caprylic acid (octanoic acid) was incorporated into various wall materials, such as urea-formaldehyde resin, melamine-formaldehyde resin, and urea-formaldehyde resin. The PCM compound was hardened with formaldehyde. The size of the capsules was measured by scanning electron microscopy (SEM) and found to be between 200 nm and 1.5 μm. This research found that the shell prepared from the urea-formaldehyde resin was the best for the caprylic acid goal. The thermal analysis determined that PCM’s melting phase change enthalpy and freezing were 93.9 J/g and 106.1 J/g, respectively [62][49].
The sol-gel method encapsulated paraffin in TiO2 as thermal energy storage materials. Paraffin in the TiO2 was encapsulated in the TiO2 according to FT-IR, XRD, and SEM measurements. The encapsulated paraffin melts at 58.8 °C and has a latent heat of 161.1 kJ/kg and solidifies at 56.5 °C with a latent heat of 144.6 kJ/kg, as the microencapsulation ratio is 85.5%.
Magnetic microcapsules were prepared using an n-eicosane core and Fe3O4/SiO2 shell, a dual-functional phase change material. After a study, the magnetic microcapsules exhibited magnetic qualities and extremely low magnetic retention and compulsion. The dual property makes them useful for innovative applications in fiber fabrics and many other military uses that require double armor properties [63][108].
Nanoencapsulated phase change materials and their high mechanical stability are used in thermal energy storage and heat transfer systems. But they have two main drawbacks; the first is poor thermal conductivity, and the second is supercooling, leaving the latent heat almost intact. To solve these problems, silicon oxide shells were prepared in a graphene microemulsion. The measurements showed an increase in thermal conductivity of 132.9%.
Microcapsules were synthesized using the microemulsion polymerization method and studied to find the best composition conditions that remained stable at high temperatures. These are n-octadecane in poly (styrene-co-divinylbenzene-co-acrylamide) shells encapsulating n-octadecane as PCM. The materials’ melting and freezing onset temperatures were slightly lower than the phase transition temperatures of n-octadecane and the enthalpy values. With this preparation technique, the capsules are stable up to 148 °C. The high-temperature stability makes them useful for temperatures above 100 °C [64][109].
The mechanical properties of the shell prepared were studied using melamine-formaldehyde resin as the shell material and found to possess a yield point of about 1.1·105 Pa, showing that the capsules exhibited plastic behavior. The capsules incorporated lauryl alcohol as phase change material with a melting point of 24 °C, and the quantity of heat included in the phase transition was 225.5 J/g. The diameter of the microcapsules varies from 5 to 10 μm, and they were spherical and smooth. The purpose of creating these capsules was to be used indoors to save energy [65][112].
