Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ahmed Fatimi and Version 2 by Rita Xu.
Different techniques have been developed to overcome the recalcitrant nature of lignocellulosic biomass and extract lignin biopolymer. Lignin has gained considerable interest owing to its attractive properties. These properties may be more beneficial when including lignin in the preparation of highly desired value-added products, including hydrogels. Lignin biopolymer, as one of the three major components of lignocellulosic biomaterials, has attracted significant interest in the biomedical field due to its biocompatibility, biodegradability, and antioxidant and antimicrobial activities.
- lignin
- chemistry
- hydrogel
- tissue engineering
1. Introduction
As a result of the increasing environmental effects caused by the fossil fuel industry, there has been an increased interest in finding alternative, clean, and globally available resources [1]. The use of lignocellulosic biomass materials can reduce dependence on petrochemical resources [2]. As evident from the literature, lignocellulosic biomass is found on top of agricultural waste, which is often likely to be disposed of by burning to generate energy [3][4][5][3,4,5]. This practice causes serious environmental problems [6]. Therefore, in recent years, lignocellulosic materials have attracted the special interest of researchers owing to their wide availability, biorenewable nature, and non-competitiveness with food [2][6][2,6]. From the environmental and economic points of view, they have proven to be good materials for the production of high-value-added products [7][8][7,8]. Lignocellulosic biomass mainly consists of 35–50 wt.% cellulose, 20–35 wt.% hemicellulose, and 10–25 wt.% lignin [9]. However, the contents of these three polymers depend on their origin, plant species, and environmental conditions [5][10][5,10]. Various processes have been developed to overcome the recalcitrant nature of lignocellulosic biomass, which is caused by the cellulose’s crystallinity, the lignin’s hydrophobicity, and the cellulose’s encapsulation by the lignin–hemicellulose network [9]. Lignin biopolymer is the principal recalcitrant component in lignocellulosic biomass, due to its very complicated structure [7], made up of propylphenolic subunits; therefore, its valorization into valuable materials is highly challenging [11]. Lignin has received a lot of interest from researchers in recent years. They have been studying the physicochemical behavior of lignin and its novel features in order to apply them to a variety of formulations [12]. Moreover, due to its key properties (e.g., biodegradability, thermal stability, reactivity, etc.), lignin is considered to be a good candidate for the development of advanced materials, including hydrogels, nanotubes, films, nanofibers, and nanoparticles, for a variety of applications [5][13][14][15][16][5,13,14,15,16].
Hydrogels are a category of soft materials that have received growing attention due to their unique properties, such as high water content, elasticity, flexibility, and biocompatibility [17][18][17,18]. In general, hydrogels can be used in a wide range of applications, such as hygiene, agricultural water retention, carbon capture, and biomedical applications [19]. The creation of such hydrogels has shifted from the adaptation of synthetic polymers to the chemical modification of biopolymers due to the latter’s biocompatibility, biodegradability, low toxicity, susceptibility to enzymatic degradation in most cases, and environmentally favorable characteristics [20]. Lignin, among other biopolymers, is a promising candidate for the development of novel hydrogels thanks to its characteristics, such as the exhibition of antioxidant and antimicrobial properties, an anti-inflammatory effect, biocompatibility, and low cytotoxicity [14][21][22][14,21,22]. In biomedical applications, lignin-based hydrogels have shown potential for uses in tissue engineering [23], wound healing [24], drug delivery [25], 3D bioprinting [26], and other applications [27][28][27,28]. For these applications, the potential of turning lignin into functional biomaterials such as hydrogels has been explored and is rapidly growing [29].
2. Lignin Biopolymer
Lignin is one of the three major components of the cell wall of lignocellulosic biomaterials [30][35]. Its name was introduced as early as 1819 by Candolle (1778–1841), derived from the Latin lignum, meaning wood [31][39]. It can be isolated from various lignocellulosic materials, including agricultural residues, energy crops, wood residues, etc. [32][33][40,41]. The molecular mass of isolated lignin ranges from 1000 to 20,000 g/mol [34][42]. Lignin serves as a structural material that adds strength, rigidity, and impermeability to plants’ cell walls and facilitates the transport of water and solutes through the vascular system [35][43]. Additionally, depending on the functional group contents, it exhibits antioxidant and antimicrobial properties, thermal stability, an anti-inflammatory effect, biocompatibility, and low cytotoxicity [21][22][21,22]. Furthermore, it plays a vital role in protecting plants against biochemical stresses by inhibiting the enzymatic degradation of other components and providing a physical and chemical barrier that protects the plant tissue from terrestrial animals and microorganisms [22]. Therefore, lignin is both biologically and mechanically suitable for the preparation of numerous biomaterials, such as hydrogels [36][44]. Despite this, the precise structure of lignin is still unknown [22].2.1. Processing Methods for Lignin Extraction and Isolation
Lignin was extracted for the first time by Bjökman in 1956 using a dioxane–water mixture [31][39]. Generally, different ways of extracting lignin from different biomass resources have been defined [37][45]. Several processes have been developed in the past to isolate lignin from other lignocellulosic biomass components, yielding different types of lignin. Reaction time, temperature, solvent concentration, and the type of raw materials are some factors that affect the extraction yield and the physicochemical properties of lignin [38][46]. The lignocellulosic biomass can undergo a preliminary step like acid hydrolysis [39][47], mechanical, or hot-water pretreatments [40][41][48,49], among others, in order to solubilize the hemicellulose fraction (Figure 1).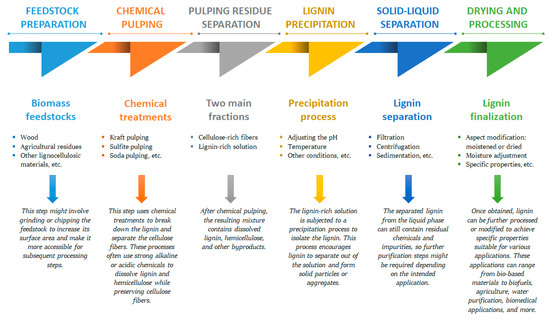
Figure 1. A typical manufacturing workflow for the processes of lignin biopolymer isolation from different biomass feedstocks.
-
Alcell® process: ethanol and solvent pulping;
-
ASAM process: alkaline sulfite anthraquinone methanol pulping;
-
Organocell process: methanol pulping followed by sodium hydroxide and anthraquinone pulping;
-
Acetosolv process: acetic acid, hydrochloric acid, or formic acid pulping.
2.2. Composition and Structure
Lignin is an amorphous, three-dimensional (3D), highly crosslinked aromatic biopolymer synthesized mainly from three primary monolignols called p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (Figure 2), which generate the different types of lignin subunits by enzymatic polymerization, namely, p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), respectively [49][57].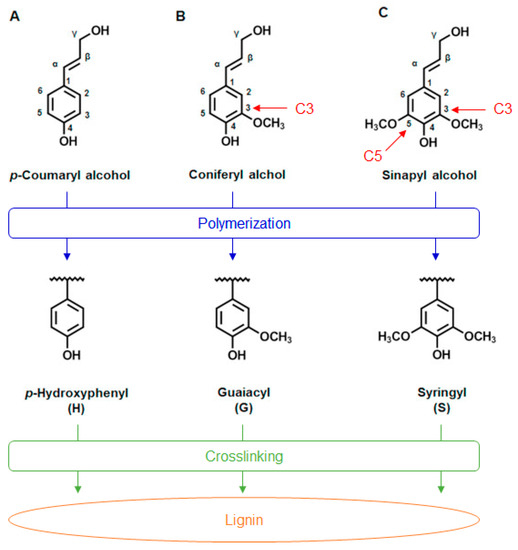
Figure 2. Chemical structures of three monolignols, which represent the precursors for the structural units in the lignin biopolymer: (A) p-coumaryl alcohol, which has no methoxy group; (B) coniferyl alcohol, which has one methoxy group at position C3; (C) sinapyl alcohol, which has two methoxy groups at positions C3 and C5. Below each chemical structure are the elemental monomers p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S).
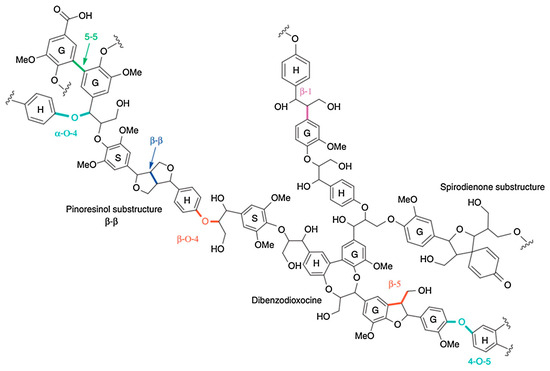
Figure 3. Example of lignin’s structure with the main linkage bonds.
2.3. Lignin Properties for Biomedical Applications
Numerous studies have highlighted lignin as a renewable, biodegradable, biocompatible, and safe biopolymer that can be applied as an antioxidant, antimicrobial, anti-ultraviolet agent, and hemostatic agent, which can be attributed to its functional groups, i.e., aromatic rings; aliphatic and phenolic hydroxyl, carboxyl, carbonyl, and methoxy groups [55][63]. This may open up new perspectives in the formulation of various materials for pharmaceutical and biomedical applications [22][35][56][22,43,64].2.3.1. Antimicrobial Activity
Various investigations have suggested that lignin could be a promising green replacement for the fossil-based agents that are useful against dangerous microorganisms [57][65]. It was reported that the methoxy and epoxy groups were responsible for the antibacterial activity of lignin, which is attained by the contact of these compounds with bacteria, which leads to damage to the cell membrane and the lysis of the bacteria [58][59][37,66]. This raised the possibility of attempts to develop high-value antibacterial lignin-based biomaterials such as hydrogels, films, nanofibers, and nanoparticles. However, the antibacterial potential of lignin depends on the preparation methods employed [60][61][67,68]. For instance, lignin nanoparticles incorporated into polylactic acid (PLA) revealed an innovative capacity to inhibit bacterial growth over time [62][69]. Additionally, in order to prevent bacterial adhesion, several lignin-based composites have been developed, such as a lignin-based hydrogel supplemented with silver nanoparticles that showed inhibitory effects on both Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) [63][64][70,71]. Another study reported that silica–lignin hybrid materials modified with nanosilver effectively inhibited the growth of Pseudomonas aeruginosa (P. aeruginosa), a dangerous human pathogen [65][72]. Additionally, lignin displayed potent antiviral activity [66][38]. For example, Qiu et al. found that lignosulfonic acid, obtained through the sulfite delignification process, showed antiviral activity against human immunodeficiency virus (HIV) and herpes simplex virus (HSV) [67][73].2.3.2. Antioxidant Activity
The authors concluded that the antioxidant activity of lignin is positively correlated with the number of phenolic hydroxyl groups and methoxy groups [57][58][37,65]. These functional groups lead to the termination of the oxidative propagation reaction via hydrogen donation [54][62]. The antioxidant capacity of lignin has been exploited in medical, pharmaceutical (e.g., anticarcinogenic agent), and polymeric applications (e.g., thermal behavior enhancement) [68][74]. The high antioxidant potential of lignin has already been mentioned in previous works, making it a good alternative to replace cytotoxic synthetic antioxidants like butylated hydroxytoluene (BHT) or butylated hydroxyanisole (BHA), which are widely used in industries such as pharmaceuticals, cosmetics, and food [69][75]. Lignin’s antioxidant activity depends on its biomass source, molecular weight, polydispersity, extraction method, and post-treatment reactions, among other things [59][70][71][66,76,77]. Lignin’s antioxidant activity was evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) [72][78]. DPPH is a stable free radical that can be used to measure the radical-scavenging activity of antioxidants. Several researchers have highlighted the antioxidant potential of lignin; for example, Toh et al. reported that the autoxidation of linoleic acid was decreased by 50% in the presence of tea leaf lignin [73][79]. Kaur et al. reported that unmodified lignin from sugarcane bagasse had higher antioxidant activity than lignin that was chemically modified via acetylation and epoxidation [74][80]. However, due to lignin’s structural variability, variation in chemical composition, and the absence of clear guidance for structure–activity relationships, its commercial applications as an antioxidant agent are limited. Therefore, it was proposed to separate lignin biopolymer into fractions with low polydispersity and well-defined properties (i.e., molecular mass, polarity, number of phenolic hydroxyl groups, and other functionalities). Despite the success of fractionation in improving antioxidant activity, lignin-derived antioxidants still cannot compete with commercial phenolic antioxidants [75][81]. Due to the antioxidant and antimicrobial potential of lignin, it can be used for a broad variety of potential applications, such as drug delivery in cancer therapy [58][62][37,69].2.3.3. Anti-Ultraviolet Capacity
Lignin is a green and ideal material that exhibits high ultraviolet-absorbent properties because of its excellent oxidation resistance [76][82]. These are attributed to the functional groups of lignin’s backbone, including chromophore functional groups such as quinones, phenolics, ketones, and conjugated double bonds [59][77][66,83]. Wang et al. described the highest ultraviolet (UV) absorption performance of soda lignin from palm fiber. Their research revealed that the branched aromatic structure of lignin contributed to the superior UV-blocking performance of palm fiber [78][84]. These properties can be exploited in the preparation of different products as an alternative to conventional petroleum-based materials, such as films for food packaging and biomedical materials [22]. Sadeghifar et al. demonstrated the significant role of lignin as a biopolymer that contains UV-absorbing functional groups to produce a renewable-based cellulose–lignin UV-light-blocking film that was stable against elevated temperatures and UV irradiation [79][85]. Additionally, lignin biopolymer can be added to commercial sunscreen products with the purpose of increasing the sun protection factor (SPF) [49][57]. Qian et al. prepared different sizes of lignin colloidal spheres via the self-assembly method and mixed them with pure skin creams in order to develop lignin-based sunscreens. The results indicated that the sunscreen performance of creams with colloidal spheres was enhanced compared with those blended with original lignin [80][86].2.3.4. Other Properties
Lignin exhibits several other critical properties that make it attractive for medical applications, including anticoagulation, cholesterol reduction, and anti-hyperglycemic effects. With the goal of developing better molecules as regulators of coagulation, several researchers have suggested that low-molecular-weight lignins (LMWLs), in polydisperse and heterogeneous preparations, mimic the polyanionic scaffold of low-molecular-weight heparins (LMWHs) [66][81][82][83][38,87,88,89]. LMWLs exhibit an anticoagulation profile in human plasma and blood that is similar to that of LMWHs [82][88]. Moreover, lignins have shown potential biological activities, such as the capability of reducing cholesterol by binding to bile acids in the intestine [84][90]. Additionally, modified alkali lignin showed significant in vitro α-amylase-inhibitory activity, thereby indicating that it potentially has anti-hyperglycemic properties and can inhibit the evolution of diabetic disease [66][38]. The critical properties of lignin biopolymer show its versatility and promise to promote healthcare. Although these features are encouraging, it is crucial to keep in mind that more investigations are required to completely comprehend the mechanisms underlying these outcomes, and to improve lignin derivatives for medical applications.3. Lignin-Based Hydrogels
By benefiting from the different attractive advantages and sustainable properties of lignin biopolymer (e.g., antioxidant and antimicrobial properties, anti-inflammatory effect, biocompatibility, and low cytotoxicity [21][22][21,22]), it can serve as an excellent building block for fabricating hydrogels [85][91] that can be widely used in the biomedical field.3.1. Hydrogels
Hydrogels are commonly known as crosslinked hydrophilic polymeric materials in the form of a 3D viscoelastic or elastic network created from natural or synthetic macromolecules [1]. The first hydrogel was synthesized by Grindlay and Clagett in 1949 [86][92]. They used a hydrogel made from poly(vinyl alcohol) (PVA) and formaldehyde as a plastic sponge prosthesis for use after pneumonectomy [86][92]. Moreover, 11 years later, a poly-2-hydroxyethylmethacrylate (PHEMA) hydrogel marked a significant turning point in the production of hydrogel materials [18]. Wichterle and Lim proposed this hydrogel as a synthetic biocompatible material useful for contact lens applications [18]. Hydrogels have attracted the significant interest of many researchers in a wide variety of applications, typically in four major sectors: biomedical [20][87][20,93], agriculture [88][94], environment [89][95], and electronics [33][41]. Biomedical applications include drug delivery, 3D cultures, tissue implants, tissue regeneration, contact lenses, and uses in healthcare products due to their unique properties such as softness, flexibility, biocompatibility, and the affinity to absorb water, organic solvents, and biological fluids up to one thousand times their own dry weight without being dissolved, due to the presence of chemical and physical crosslinking in their structure [18][85][90][18,91,96]. They stay in balance in an aqueous environment due to the balance between the elastic forces of their 3D interconnected structure and the osmotic forces of the surrounding liquid (Figure 4) [20]. Other interesting properties of these materials include their ability to control the diffusion process and to perform dramatic volume transitions in response to specific environmental stimuli, which can be physical, chemical, or biological [91][92][97,98]. Hydrogels restore their original state when these environmental stimuli disappear [93][99]. They can be characterized by numerous physical parameters, such as size, elastic modulus, swelling, and degradation rate [90][96]. These materials can be classified according to their origin, preparation method, crosslinking nature, swelling capacity, and sources. However, classification based on the nature of crosslinking is the major factor that determines whether a hydrogel is chemically or physically crosslinked [92][98]. Chemically crosslinked networks involve covalent bonding that is permanent at junctions; this gives the final product more resistant mechanical properties. This type of hydrogel requires a crosslinking agent and free-radical polymerization in the presence of initiators [33][41]. Meanwhile, physical networks have reversible junctions that arise from either polymer chain entanglements or physical interactions such as ionic interactions, hydrogen bonds, hydrophobic forces, or Van Der Walls interactions, without any crosslinking agent [1][33][1,41]. Depending on the polymer source, hydrogels can be synthetic, natural, or hybrid [94][100]. Natural hydrogels, using biopolymers as building blocks, have beneficial properties that are favored by researchers in terms of biocompatibility, cost-effectiveness, and biodegradability. This is the case with lignin-based hydrogels, which are considered to be a more sustainable and environmentally friendly alternative to synthetic hydrogels [85][91] and are incredibly appreciated throughout biomedicine [95][101].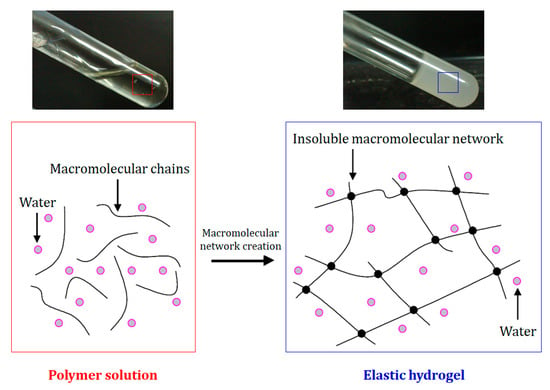
Figure 4. A schematic illustration of the principle of hydrogel formation based on hydrogel crosslinking, which forms an insoluble macromolecular network in the environmental fluid: The macromolecular network’s creation could be based on physical or chemical crosslinking.
3.2. Preparation of Lignin-Based Hydrogels
Lignin is an attractive raw material for hydrogel preparation, with significant sustainability and green chemical connotations [96][103]. Using lignin biopolymer alone to form a hydrogel cannot ensure good water retention and high mechanical properties; therefore, it is necessary to resort to copolymerization in order to obtain a better performance in these properties [97][30]. These methods can be divided into two major groups: lignin-based hydrogels formed by chemical interactions, and lignin-based hydrogels formed by physical interactions. More precisely, chemically crosslinked hydrogels can be obtained by grafting, free-radical polymerization, click chemistry, enzymatic reactions, thermo-gelation, and radiation crosslinking. On the other hand, to create physically crosslinked hydrogels, changes in intermolecular interactions—such as ionic crosslinking, hydrophobic interactions, and hydrogen bonding—have been employed. Both chemical and physical crosslinking can be used to form hydrogels [90][96]. These processes can be initiated by temperature, ionic, gamma, or redox pairs [1].3.2.1. Lignin-Based Hydrogels by Chemical Interactions
Due to the chemical reactivity of lignin, most lignin-based hydrogels described in the literature were synthesized by chemical crosslinking [98][116]. The crosslinking of grafted lignin and monomers is a way to synthesize hydrogels, in which lignin and unsaturated monomers or functional compounds are grafted onto the main chain by the esterification reaction of lignin’s phenolic groups. The grafted lignin is then crosslinked with other unsaturated monomers, such as hydroxyethyl acrylate, to form hydrogels with good water retention [85][91]. Ma et al. used this approach to synthesize lignin-based hydrogels that could be used to remove lead ions from the human body because of their toxicity and their potential effects on the nervous, immune, renal, reproductive, and cardiovascular systems [99][117]. Other hydrogels were prepared by copolymerization of acrylic acid and other functional compounds such as organo-montmorillonite (OMt) with grafted lignin, which was synthesized by adding lignin in the presence of sodium hydroxide, with N,N’-ethylenebisacrylamide (NEBA) and ammonium persulfate (APS) as initiators [85][91]. Moreover, a novel lignin-based thermosensitive gel was prepared by thermal polymerization of phenolated alkali lignin and N-isopropyl acrylamide. These materials can be used to control pests and diseases. They can also release and recover chemicals through temperature changes in nature. In addition, lignin has the function of absorbing UV rays; this property has a good preservation effect for UV-decomposable drugs [100][118]. Sathawong et al. [101][107] recovered lignin from kraft black liquor by the acidic precipitation method and synthesized a lignin–agarose hydrogel with epichlorohydrin (ECH) as a crosslinking agent. Agarose is a natural polysaccharide extracted from the cell walls of certain Rhodophyceae algae. Even at low concentrations, it enhances system stability, making it suitable for hydrogel scaffolds, cartilage, neural tissue, and wound healing. Kraft lignin was also mixed with solid phenol and an aqueous solution of formaldehyde to form highly porous hydrogels [102][32]. Teng et al. [103][104] prepared a new lignin-based hydrogel from lignin amine and poly(ethylene glycol) diglycidylether (PEGDGE). Lignin amine was prepared via the Mannich reaction by grafting the amino groups onto sodium lignin sulfonate. These hydrogels showed superior swelling capacity, viscoelasticity, and shear properties [103][104]. The aminated lignin was also expected to react with poly(vinyl alcohol) (PVA) to form a hydrogel, in which silver nanoparticles were reduced in situ to enhance the antimicrobial properties of lignin against S. aureus and E. coli [64][71]. Lignosulfonate was used by Wu et al. [104][109] to prepare a superabsorbent hydrogel through graft copolymerization of magnesium lignosulfonate with acrylic acid and acrylamide, using potassium persulfate as an initiator and N,N’-methylenebisacrylamide (NMBA) as a crosslinker. PVA is a hydrophilic, semicrystalline synthetic polymer that exhibits attractive properties such as a high degree of swelling, a rubbery or elastic nature, non-toxicity, and bioadhesivity, which make it a good candidate for the synthesis of hydrogels for biomedical applications. PVA was mixed with lignin or lignin–epoxy resins and ECH crosslinking agents to form hydrogels by covalent crosslinking [8]. Lignin–PVA hydrogels were also prepared by Wu et al. [105][110]. The lignin macromolecule was incorporated into the PVA hydrogels in the presence of ECH as a crosslinker to form a doubly crosslinked network exhibiting swelling ratios of up to 456 g/g, excellent water retention, and biodegradability. Wu et al. then proposed a structural mechanism for the lignin–PVA hydrogel (Figure 5). In this case, the hydroxyl group of PVA or lignin reacted with the epoxy group of ECH to form an ether bond, while hydrochloric acid was taken out of the other end of ECH to generate a new epoxy group. The previously mentioned reaction was carried out until the crosslinking process was finished, at which point the extra crosslinking agent was eventually changed into glycerol [106][119]. Additionally, the hydroxyl groups of lignin and PVA generated potent intermolecular hydrogen bonds [107][120], and PVA was able to crosslink with ECH to form a compact network structure. Finally, through ECH, lignin–PVA and PVA–PVA might crosslink with one another to form the lignin–PVA hydrogel [105][110].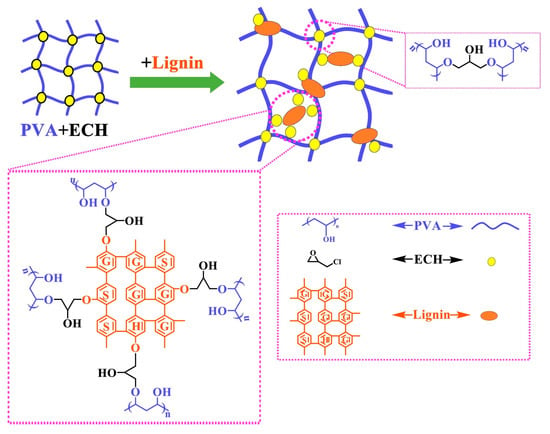
Figure 5. The structural mechanism of the lignin–PVA hydrogel: Through ECH, lignin–PVA and PVA–PVA might crosslink with one another to form the lignin–PVA hydrogel.
3.2.2. Lignin-Based Hydrogels by Physical Interactions
In order to avoid the use of toxic chemical reagents, physical crosslinking methods are used for lignin hydrogel preparation as a greener and more economical approach [116][122]. Oveissi et al. [24] used lignin for additional crosslinking of hydrophilic polyether-based polyurethane (HPU) hydrogels in order to improve their mechanical strength and processability by forming hydrogen bonds between the PU and the polar sites of lignin’s backbone. The authors confirmed that hydrogen bonding was the dominant toughening mechanism, and they elucidated the toughening mechanism by applying the Lake–Thomas and sequential deboning theories. Finally, these hydrogels have demonstrated good biocompatibility with primary human dermal fibroblasts and have been proposed for scalable fabrication methods such as 3D printing, fiber spinning, and film casting [24]. Another preparation method for lignin-based hydrogels was successfully introduced by Ravishanker et al. [23], who prepared a physical hydrogel with greater viscoelastic properties by mixing an aqueous–acidic solution of chitosan with alkali lignin, making it highly desirable for application as scaffolds in tissue engineering and wound healing. The incorporation of lignin could potentially improve the shear strength and viscosity of chitosan [23]. Lignin was also used as a physical crosslinking agent for the preparation of a lignin–chitosan–PVA composite hydrogel [117][115]. SIn this study, sulfonate groups in the chemical structure of lignin formed ionic bonds with the amino groups of chitosan, thereby increasing the mechanical strength of the hydrogel. The developed hydrogel showed excellent biocompatibility, antimicrobial activity, and high mechanical strength. Zhang et al. confirmed that lignin–chitosan–PVA hydrogels exhibit potential for a wide range of medical applications, such as packaging expensive drugs [117][115]. Figure 6 shows the crosslinking mechanism of lignin–chitosan–PVA hydrogel, in which active groups of lignin such as hydroxyl, carboxyl, and sulfonic groups can form hydrogen bonds (formed by the hydroxyl of PVA and the hydroxyl of lignin) and ionic bonds (formed by the amino group of chitosan and the sulfonic group of lignin) [117][115].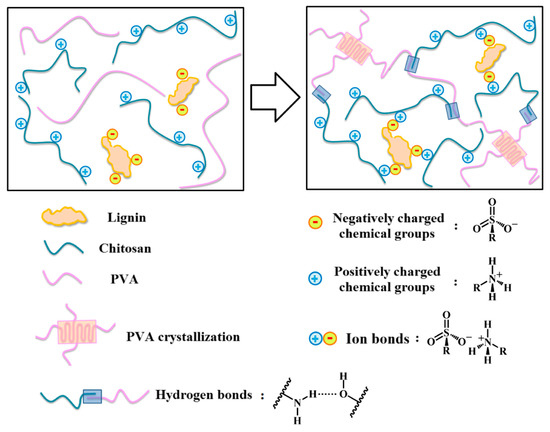
Figure 6. The crosslinking mechanism of lignin–chitosan–PVA hydrogel: active groups of lignin, such as hydroxyl, carboxyl, and sulfonic groups, can form hydrogen bonds (with groups in the chemical structures of chitosan and/or PVA) and ionic bonds (with groups in the chemical structures of chitosan and/or PVA).
