Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mahmoud Kandeel and Version 2 by Catherine Yang.
Mesenchymal stem cells (MSCs) are a type of versatile adult stem cells present in various organs. These cells give rise to extracellular vesicles (EVs) containing a diverse array of biologically active elements, making them a promising approach for therapeutics and diagnostics.
- mesenchymal cell
- extracellular vesicles
- neurodegenerative diseases
- treatment
1. Introduction
Mesenchymal stem cells (MSCs) are a type of adult stem cell with the ability to develop into different types of mesoderm-derived cells. MSCs can be located in various tissues like bone marrow, adipose tissue, umbilical cord, dental tissue, connective tissues of muscle and skin, and endometrial polyps [1][2][1,2]. Traditionally, MSCs were not considered to be naturally present in the brain; however, recent studies have indicated that they might exist as perivascular cells in nearly all adult tissues, including the brain [3]. MSCs can be categorized into two main groups, namely embryonic or adult, depending on the specific developmental phase from which they are derived [4]. These cells possess two crucial characteristics: the capacity to transform into different cell lineages and the ability to renew themselves. As multipotent stem cells, they show great potential in preclinical investigations for treating diverse medical ailments [5].
2. MSC-EVs in Alzheimer’s Disease
AD is a kind of dementia that typically affects the elderly. AD is a progressive neurological illness and the most frequent cause of dementia [6][134]. Damage is pervasive in AD, as many neurons cease to function, lose connections with other neurons, and die, affecting activities critical to neurons and their networks, including communication, metabolism, and repair. Initially, AD results in the deterioration of neurons and their synaptic connections in the brain regions associated with memory. As the disease progresses, it also impacts the regions of the cerebral cortex responsible for language, reasoning, and social interactions [7][135].
This neurodegenerative condition gradually and irreversibly impairs brain functioning (remembering, reasoning, and thinking), thought content, personality, and behavior [8][136]. The prevailing scientific explanations for the pathological features of AD involve the following processes: accumulation of Aβ outside cells, the creation of neurofibrillary tangles (NFT) caused by the buildup of hyperphosphorylated tau inside cells, and persistent neuroinflammation. These factors are considered to be the primary contributors to the development and progression of AD [9][10][137,138]. Modulation of the aberrant gene expression in AD can effectively improve the cognitive response in animal models of AD [11][139]. Neurotic communication abnormalities and the loss of individual neurons are caused by aberrant protein accumulations outside and inside nerve cells [10][138]. The first phases of AD pathogenesis are assumed to be caused by the deposition of Aβ, the primary component of amyloid plaque, within neurons [12][140]. These findings underline the importance of exosomes in the progression of AD through the spread of amyloid plaques [13][141]. In addition, the content of exosomes can modulate the gene expression of AD-associated genes.
Several research investigations have explored the use of exosomes as a potential biomarker for the early detection of AD (Figure 14). Additionally, they have been investigated as a means to transport therapeutic agents, such as small chemical molecule drugs, miRNA, and siRNA [14][15][142,143]. For instance, Saman et al. employed tau-containing exosomes generated from CSF for the initial diagnosis of AD [16][144]. Furthermore, since CSF-derived exosomes contain both p-tau and Aβ, the discovery of both possible biomarkers in exosomes may indeed raise the value of the currently employed marker for the initial detection of AD [17][18][145,146]. In this regard, using a combination of markers to diagnose AD enhanced specificity and sensitivity by 86% [19][147].
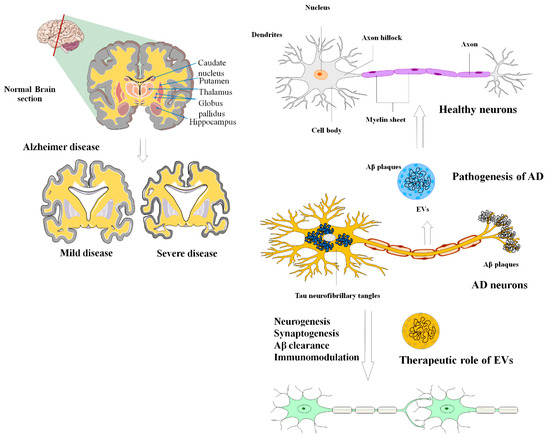
Figure 14. The applications and uses of MSCs-EVs in the diagnosis and treatment of AD. The most widely accepted scientific explanations for the pathogenic hallmarks of AD are extracellular aggregation of beta-amyloid peptide (A), formation of neurofibrillary tangles due to intracellular deposition of hyperphosphorylated tau, and persistent neuroinflammation. EVs can effectively alleviate these negative circumstances during the development of AD.
The AD sufferers’ CSF-derived exosomes had greater levels of miR-598 and miR-9-5p than their healthy counterparts [20][148]. Plasma, on the other hand, has long been evaluated to distinguish markers; hence, plasma seems to be a more convenient and accessible option [21][149]. Furthermore, the biological components of exosomes have demonstrated great accuracy in the initial detection of AD [22][150]. When comparing plasma exosomal protein expression among AD cases and healthy controls, researchers discovered that patients with AD had higher levels of neuron-derived proteins such as Aβ and tau [18][146].
Several investigations have confirmed the neuroprotective properties of neuron-derived exosomes. Exosomes generated from glia, for instance, protect neurons from oxidative stress [23][151]. According to the findings of a study into the processes involved with exosomes in Aβ clearance, neuron-derived exosomes injected into the brains of AD transgenic mice aided in Aβ peptide elimination. Because of conformational changes, the prion receptor on the exosomal surface can bind to amyloid plaques and convert them to harmless forms. Exosomes also hasten the absorption of Aβ extracellular plaque by microglia.
Exosomes released by MSCs obtained from connective tissues such as bone marrow and adipose tissue not only effectively traverse the blood–brain barrier (BBB) but also successfully degrade both intracellular and extracellular Aβ peptides in the brain. This degradation is attributed to the presence of neprilysin enzymes [24][25][152,153]. MSC-EVs directly interact with Aβ through their lipid membranes, promoting the clearance of Aβ plaques by microglia. Additionally, MSC-EVs transport neprilysin, an enzyme that breaks down Aβ, thereby indirectly reducing the accumulation of Aβ inside cells. In vivo studies investigating the therapeutic effects of MSC-EVs in animal models of AD have predominantly employed long-term treatment regimens lasting for weeks or months. The administration of EVs in these studies has been done either systemically (via the bloodstream) [26][154] or intracerebroventricular [27][28][155,156], demonstrating either partial recovery [26][27][154,155] or a protective function in diminishing the burden of Aβ plaque and the number of dystrophic neurites [28][156].
The injection of MSCs or MSC-EVs into hippocampal neurons enhances their resilience against the synaptic degradation caused by Aβ and the harmful effects of oxidative stress [29][157]. The results proposed several potential mechanisms to explain this phenomenon, including the decreased presence of extracellular Aβ due to the heightened endocytic capability of MSCs, the secretion of EVs containing antioxidant enzymes like catalase, and the paracrine activity resulting from the eventual release of trophic factors and anti-inflammatory cytokines such as VEGF, IL-6, and IL-10. Although most studies on AD treatment have primarily employed MSC-EVs, recent investigations suggest that different sources of stem cells possess therapeutic potential in combating AD-related cognitive disorders through distinct processes, such as reducing the extracellular and intracellular deposition of Aβ oligomers [29][157].
Although MSC-EVs hold promise for treating AD, their full potential is hindered by several challenges. These issues include inadequate targeting efficiency, inconsistent treatment results, and limited production yield [30][160]. To overcome these limitations and enhance the effectiveness of MSC-EVs as AD treatments, it is essential to functionalize and engineer the EVs structures. Various methods can be employed for this purpose, such as preconditioning the parental cells to improve the natural treatment’s effectiveness, incorporating therapeutic cargo or drug loading into MSC-EVs, modifying the surface of MSC-EVs to enhance targeting capabilities, and utilizing artificial MSC-EVs to scale up production [31][161].
MSC-EV-based AD treatments were accomplished using different types of mesenchymal stem cells: bone marrow-derived MSCs (mBMSCs), adipose-derived MSCs (mADSCs), and human umbilical cord-derived MSCs (hUCMSCs). To enhance the therapeutic efficacy of MSC-EVs, they are modified with specific peptides like the rabies virus glycoprotein (RVG) peptide or loaded with miRNAs like miR-29 and miR-22 [31][161]. MSC-EVs were administered through various routes, including intravenous (IV), intracranial injection (IN), and dorsal hippocampus injection. IV administration of RVG-modified MSC-EVs has shown promise in improving learning and memory by reducing Aβ deposition and astrocyte activation while promoting the production of anti-inflammatory factors. Similarly, dorsal hippocampus injection of miR-29-loaded MSC-EVs reduces BACE1 expression and activates PKA/CREB, leading to improved cognitive function [32][162]. Preconditioning MSCs in an AD environment before administration is another promising strategy. Hypoxic preconditioning of mADSCs shifts microglial M1/M2 polarization, reduces inflammatory factors, and upregulates TREM2 expression, thereby improving cognitive function [33][163].
Although most studies have used MSC-EVs for AD treatment, it is worth noting that various stem cell sources have demonstrated therapeutic potential in alleviating AD-associated cognitive deficits via multiple mechanisms, such as reducing extracellular and intracellular oligomer deposition (Table 14).
Table 14.
The use of extracellular vesicles produced from stem cells in neurodegenerative disorders.
| Disease | Type of EVs and Origin | Outcomes | Ref |
|---|---|---|---|
| AD | MSCs/exosomes | Enhances neurogenesis, reduces Aβ, and the restoration of cognitive function. | [34][35][164,165] |
| PD | MSCs/exosome | Transferring of the miR-133b regulates neurite outgrowth. | [36][166] |
| Improved neuronal function and oligodendrogenesis stimulation | [37][101] | ||
| Reduction in α-syn aggregates | [38][167] | ||
| MS | MSCs/exosomes MSCs/EVs | Drive peripheral resistance, activate apoptotic signaling pathway in self-reactive lymphocytes, and stimulate regulatory T cell differentiation by - IL-10 and TGF-β secretion - expression of PD-L1 and TGF-β |
[39][168] |
| MSCs/exosomes | Reduce CNS inflammation and demyelination by performing the following: - Shifting microglial polarization toward an M2 phenotype |
[40][41][169,170] |
3. MSC-EVs in Parkinson’s Diseases
PD is the second most prevalent neurodegenerative disease worldwide, originally discovered by James Parkinson in 1817. While the exact causes of PD are still unknown, both genetic and environmental factors contribute to its development [42][43][171,172]. Degeneration of dopaminergic neurons and impairment of dopamine production in multiple dopaminergic networks characterize the disease. The formation of Lewy bodies in the neurological system, which are protein clumps formed of α-synuclein (α-syn), is linked to the death of dopaminergic neurons and the disturbance of their normal functioning. The nigrostriatal pathway, which includes the substantia nigra pars compacta and the striatum, is the most damaged [44][173]. There is currently no cure for PD, but researchers are exploring various treatment options, including the use of MSCs and their EVs [45][46][47][48][174,175,176,177].
Exosomes can transport enzymatically active proteins like phosphatase and tensin homolog (PTEN) and biologically active lipids, such as prostaglandins, to specific target cells [49][50][178,179]. Within exosomes, there are various proteins referred to as “exosome markers,” which are primarily involved in their formation. These exosomes also carry transmembrane molecules that assist in the immunoselection process, enabling the identification of exosomes with a specific biological origin and enhancing their sensitivity as biomarkers. In the context of NDs like PD, exosomes have been found to contain misfolded proteins such as α-syn [51][52][180,181].
The presence of genetic material, such as miRNAs, is one of the largest common contents of exosomes [53][74]. Disorders like PD exhibit considerable disruption in gene expression, especially at the miRNA level [54][55][182,183]. Exosomes derived from MSCs can transfer miRNAs to neuronal cells. Notably, exosomes rich in miR-133b have been found to promote neurite outgrowth, which is advantageous for PD since this particular miRNA is generally suppressed in PD cases [36][166]. However, it should be noted that MSC-derived exosomes also contain miR-143 and miR-21, which are known to play significant roles in regulating immune responses and contributing to neuronal loss associated with chronic inflammation [56][184].
The α-syn can be released either directly into the extracellular space or enclosed within exosomes [57][185]. Furthermore, α-syn has been observed to engage with synaptic vesicles, leading to an augmentation of neurotransmitter release and assisting in the assembly of SNARE proteins. Typically, synaptic vesicles that contain α-syn are sorted into early endosomes either through Golgi or clathrin-mediated endocytosis [58][59][186,187]. The endosomes that contain α-syn progress and develop into multivesicular bodies (MVBs). These MVBs eventually merge with the cell membrane and release their contents as exosomes, aided by VPS4 and small ubiquitin-like modifier (SUMO) proteins. Another possibility is that the α-syn-containing endosomes are directed towards recycling endosomes, where they are released from the cell as secretory granules through a process dependent on Rab11a [60][61][188,189]. While engaged in these processes, the level of calcium in the cytoplasm governs the discharge of α-syn from viable cells. Although the quantity of α-syn found in exosomes is limited, recent studies propose that exosomes create a favorable setting for the aggregation of α-syn, potentially contributing to the propagation of PD (Figure 25). The toxic variant of α-syn, known for its ability to trigger neuronal cell demise, is typically recognized as oligomeric α-syn [62][63][190,191].

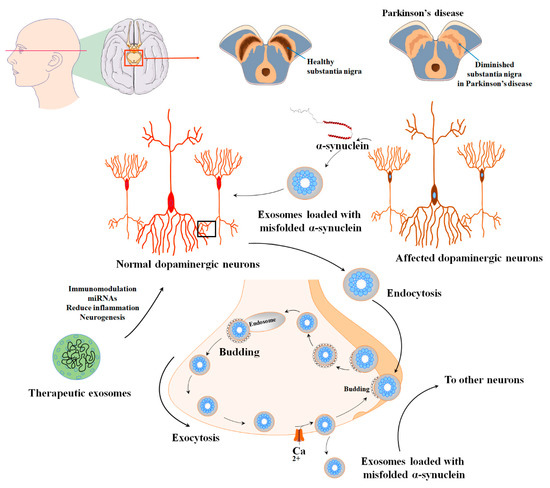
Figure 25. The various applications and uses of extracellular vesicles derived from mesenchymal stem cells in the diagnosis and treatment of PD. PD is characterized by the degeneration of dopaminergic neurons and impaired dopamine production within multiple dopaminergic networks. This condition is linked to the formation of Lewy bodies in the nervous system, which are aggregates of α-synuclein protein and result in the degeneration of dopaminergic neurons and disruption of their normal functioning. The nigrostriatal pathway, consisting of the substantia nigra pars compacta and the striatum, is particularly affected in PD. α-syn-containing synaptic vesicles are usually sorted into early endosomes through Golgi or clathrin-mediated endocytosis. However, extracellular vesicles show promise in effectively alleviating these detrimental conditions during the pathogenesis of PD.
Currently, the identification of PD primarily relies on the observation of visible motor symptoms during clinical examination. Unfortunately, there are no reliable diagnostic methods available for detecting PD in its early stages. Developing a technique for early detection would be a significant advancement in the field. Previous studies have indicated that certain components of EVs, such as exosomes obtained from the blood or CSF of PD patients, can serve as effective biomarkers for the disease [64][65][66][192,193,194]. It is crucial to comprehend the complexity of exosomes derived from MSCs and how their miRNA contents interact with the cellular and molecular pathways associated with PD.
MSC-EVs have been proposed as a promising therapeutic tool for PD, as they can act as a vehicle for the delivery of therapeutic molecules, such as miRNAs, to the brain [45][174]. MSC-EVs could be modified using molecular engineering techniques to carry protein and RNA cargoes, making them a promising therapeutic option for PD [46][175]. MSC-derived secretome treatment has shown encouraging results in experimental models of PD [47][176].
MSCs and the EVs they produce have been suggested as a viable treatment approach for various neurodegenerative conditions such as PD. This is because they possess the capability to support the survival of dopaminergic neurons, encourage the formation of new neurons, decrease neuroinflammation, and improve overall functional recuperation in animal models [48][177]. In a pilot investigation, individuals diagnosed with progressive supranuclear palsy (a rare and serious type of parkinsonism) received mesenchymal stem cells derived from bone marrow. These cells were administered through the cerebral arteries. The results showed that all of the treated patients survived for a year following the infusion of the cells, except for one patient who passed away nine months later due to reasons unrelated to the delivery of cells or the progression of their illness [67][195].
One of the major obstacles to PD treatment is access to the damaged cells. However, recent bioengineering research has led to the production of genetically modified cells with improved therapeutic efficiency. Improved adhesion, migration, and survival are new methods for not only preserving but also increasing the biological properties and therapeutic potential of MSCs [68][196]. MSCs that have been genetically designed to produce specific neurotrophic factors, such as brain-derived neurotrophic factors, or MSCs that have been modified to boost their survival and ability to migrate toward the lesion location. Concerning PD, various studies have utilized engineered MSCs expressing vascular endothelial growth factor, tyrosine hydroxylase, or modified to enhance the production of cerebral dopamine neurotrophic factor or glial cell-derived neurotrophic factor. These experiments have shown promising outcomes in preclinical rodent models [69][197]. Furthermore, genetically engineered EVs showed promising results in PD animal models. When subjected to catalase-loaded EVs in a cell culture environment, macrophages that were activated with lipopolysaccharide (LPS) and tumor necrosis factor (TNF) demonstrated decreased levels of reactive oxygen species (ROS). In a mouse model of PD using 6-OHDA, administering catalase-loaded EVs resulted in reduced activation of microglia compared to the application of free catalase. These findings suggest that delivering catalase through EVs has the potential to effectively decrease oxidative stress and neuroinflammation in PD [70][198]. Interestingly, the introduction of dopamine-loaded EVs led to a remarkable 15-fold increase in dopamine distribution within the brain. This heightened distribution not only resulted in improved therapeutic effectiveness but also significantly reduced systemic toxicity compared to the administration of free dopamine. These findings suggest that utilizing EV-based drug delivery holds promising potential as a viable and effective treatment option for PD [71][199].
4. MSC-EVs in Multiple Sclerosis
MS is inflammatory demyelination of the central nervous system [72][200] (Figure 36). In addition to inflammation and demyelination in the spinal cord and brain, other pathological biomarkers of MS include BBB disruption, reactive gliosis, oligodendrocyte loss, and neuron and axonal degeneration [73][201]. According to the National MS Society, the four major types of MS are primary progressive MS (PPMS), secondary progressive MS (SPMS), relapsing–remitting MS (RRMS), and clinically isolated syndrome (CIS) (NMSS) [74][202].

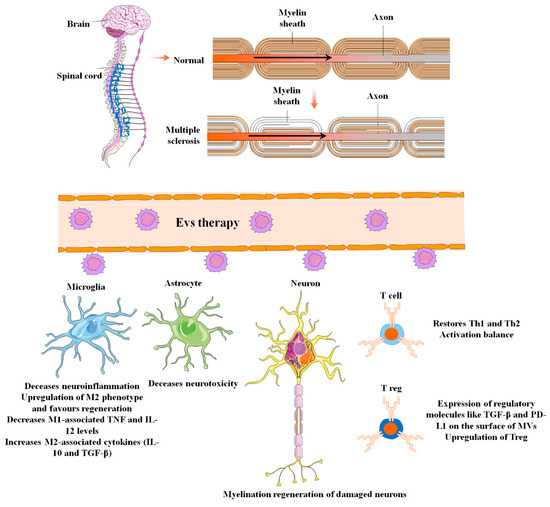
Figure 36. The applications and uses of mesenchymal stem cell-derived extracellular vesicles in the diagnosis and treatment of multiple sclerosis (MS). MS is central nervous system demyelination caused by inflammation. Other pathological biomarkers of MS, in addition to inflammation and demyelination in the spinal cord and brain, include BBB disruption, reactive gliosis, oligodendrocyte loss, and neuron and axonal degeneration. EVs can effectively relieve these adverse conditions during MS pathogenesis through several mechanisms of action.
Autoimmune attacks are the most significant reason for axon demyelination in illnesses like MS. Ideally, tissue restoration via stem cell transplantation may lead not only to axon reconstruction by replacing lost and destroyed cells but also to anti-inflammatory and paracrine neuroprotective effects, potentially preventing progressive neural and axonal degeneration [75][76][203,204].
The exosomes derived from periodontal ligament stem cells and present in the conditioned medium demonstrated anti-inflammatory and suppressive effects in mice models of MS called experimental autoimmune encephalomyelitis (EAE). The study indicated that the exosomes promoted significant remyelination in the spinal cord and reversed the progression of MS by increasing the levels of anti-inflammatory cytokines, specifically IL-10. Moreover, the impaired activation of T cells, which is a key factor in regulating the balance between T helper (Th)1 and Th2 cells, was identified as one of the pathological features of MS [77][205].
The EVs produced by placental-derived MSCs (PMSCs) could achieve therapeutic effects similar to individual EAE therapy if administered in high doses. Also, according to VEGF proteomic analyses, the HGF was found in EVs produced from PMSC. PMSCs regulate the immune system by triggering regulatory T cells (Tregs) with large amounts of these substances that they release. This discovery indicated that PMSC-EV can stimulate myelin regeneration and elicit immunomodulatory effects comparable to PMSC therapy in the EAE mice model [41][170]. Likewise, the administration of MSC-EVs obtained from adipose tissue of humans through intravenous treatment improves the condition of animals with EAE. This is achieved by inhibiting the infiltration of immune cells, modulating their activation, and reducing the secretion of inflammatory cytokines [78][206].
Induction and maintenance of immunological tolerance is a major goal in the treatment of autoimmune conditions. Maintenance and progression of regulatory molecules like TGF-β, programmed death ligand-1 (PD-L1), and galectin-1 via biological interventions in the host immune system are one of the most current approaches for peripheral tolerance [78][79][206,207]. The ability of MSC-MV to enhance environmental resilience in splenic mononuclear cells (MNCs) from mice with EAE was investigated. The MVs derived from MSCs trigger apoptotic signaling in self-reactive lymphocytes, prompting them to release IL-10 and TGF-β. Additionally, they upregulate the expression of regulatory molecules like TGF-β and PD-L1 on the surface of MVs, which promotes the differentiation of regulatory T cells (Tregs). This ultimately contributes to the development of peripheral immune tolerance [39][168].
A different developing approach to induce immunological tolerance in individuals with MS involves directing microglia to adopt the M2 phenotype [80][208]. Microglia are the CNS’s resident macrophages that are rapidly activated by microenvironments (like infection, ischemic injury, and pro-inflammatory cytokines such as TNF-α and IL-1β) to make a differentiation either into the M1 phenotype, which causes CNS damage and generates pro-inflammatory cytokines, or the M2 phenotype, which fosters tissue regeneration by producing anti-inflammatory cytokines [81][209].
In the early stages of MS, an imbalance of M1/M2 macrophages and a shift toward pro-inflammatory M1 phenotypes was considered to be one of the major drivers of tissue injury in the CNS. As a result, it is thought that prompting microglia to polarize toward the M2 phenotypes might improve MS patients’ neurological symptoms [80][208]. In this regard, Li et al. investigated the impact of BMSC paracrine pathways, namely exosome mediation, on microglial polarization and motor functional improvements in an EAE mouse model [40][169]. They found that exosomes derived from BMSC can decrease demyelination and inflammation of the CNS while improving neural behavioral ratings in the EAE animal model via shifting the polarity of microglia toward an M2 phenotype. Furthermore, MSC exosome therapy decreased M1-associated TNF and IL-12 levels while increasing M2-associated cytokines (IL-10 and TGF-β) [40][82][169,210].
EVs derived from MSCs of adipose tissue promote recovery from demyelination in an animal model for progressive MS, and lab animals induced recovery from demyelination and curation of brain atrophy [83][104]. MSC- EVs have the potential to exert positive effects by transporting crucial molecules, including DNA, enzymes, proteins, mRNA, ncRNAs, and different ligands, to the intended recipient cells [84][211]. The field of molecular engineering has made alterations to EVs by incorporating myelin antigens. This modification transforms EVs into platforms capable of presenting antigens, thereby enabling the restoration of antigen-specific peripheral immune tolerance in autoreactive T cells. This innovative technique can be regarded as a groundbreaking “EV-based vaccine” that holds significant potential in the treatment of MS by reinstating immune tolerance. Antigen-presenting EVs could decrease harmful immune reactions while preserving the integrity of the remaining immune system, thereby minimizing the likelihood of adverse outcomes [84][211].
Exosomes can carry medications to MS patients because of their capacity to cross the BBB. Diverse functional elements on the surfaces of exosomes, such as aptamers and antibodies, dramatically improve the exosomes’ specificity [85][212]. Based on these findings, it’s safe to say that MSC-EVs will represent the future of the MS therapy approaches for various reasons, such as their safety and capacity to cross the BBB.
The therapeutic effects of genetically engineered MSCs in different MS models were investigated. Female mice were treated with Mouse MSCs expressing the Mouse IFN-β gene. Intravenous administration of engineered MSCs resulted in increased Tregs and IL-10 production while reducing inflammatory cell infiltration, suggesting potential therapeutic benefits for MS [86][213]. Furthermore, treatment with human BM-MSCs engineered to express PSGL-1, FUT-7, and IL-10 resulted in an increase in clinical score and myelination, coupled with reduced inflammatory infiltration. However, the observed impact of the engineered MSCs on MS pathogenesis appears complex, necessitating further research for a comprehensive understanding [87][214].
5. MSC-EVs in Amyotrophic Lateral Sclerosis
ALS is a fatal neurodegenerative condition that typically develops in adulthood and was first identified in the 1870s. While around 5% to 10% of people with ALS have a family history of the disease (known as familial ALS or fALS), the remaining 90% to 95% of cases (referred to as sporadic ALS or sALS) do not seem to have a clear genetic connection [88][215]. The patient’s condition worsens and becomes life-threatening within a period of 2 to 5 years after the disease begins. Both sporadic sALS and familial fALS have a shared characteristic of experiencing a targeted loss of upper motoneurons in the primary motor cortex, as well as lower motoneurons in the brainstem and spinal cord. However, the disease does not affect specific motoneurons that control pelvic muscles and eye movements. The exact reason for this differing vulnerability of motoneurons is presently unknown [89][216].
Neurodegeneration is characterized by a complex underlying mechanism involving various pathways. One such pathway involves the increased entry of calcium ions (Ca2+) into motoneurons, which is triggered by elevated levels of the neurotransmitter glutamate in the synaptic cleft (known as glutamate excitotoxicity) caused by dysfunction in the uptake process by astrocytes. Due to problems with mitochondrial function, the concentration of Ca2+ remains elevated within the cytoplasm, leading to the activation of enzyme pathways dependent on Ca2+ and contributing to oxidative stress, potentially leading to dementia.
Exosomes and MVs have received increased attention as boosters and suppressors of disease processes due to their ability to transmit biological information across large distances. Exosomes derived from primary neurons or neuroblastoma have been shown to improve the course of AD in a mouse model by sequestering intracerebral substances. After oxidative stress, the neuroprotective effect of exosomes produced from adipose-derived stromal cells (ASC) was also established in primary murine hippocampus neurons and human neuroblastoma cells. Exosomes derived from BM-MSC have also been used to aid recovery and neuroregeneration following strokes and traumatic brain injuries. Exosomes derived from ASC have recently been shown to protect neurons in an in vitro model of ALS [90][91][92][16,217,218].
The NSC-34 cell line, which mimics motoneurons affected by ALS, was genetically modified with different SOD1 point mutations to replicate the characteristics of the disease. In the research, H2O2 was utilized as a harmful stimulus. The lifespan of ALS motoneurons was enhanced by exosomes, which inhibited the apoptotic pathway. This suggests that exosomes have the potential to be utilized as a therapeutic approach for ALS. Another investigation demonstrated that exosomes derived from ASCs could potentially treat ALS by reducing the presence of mutant SOD1 and enhancing the functioning of mitochondrial proteins involved in aggregation [93][219]. Although the researchers still have a long path ahead of them, the recent discovery of EVs gives patients with ALS new hope and should stimulate more research in this approach.
Using EVs derived from MSCs in ALS treatment has several advantages over using MSCs themselves. EVs can cross the blood–brain barrier, which is a significant challenge for MSCs [94][220]. EVs can be stored and transported more easily than MSCs [95][221]. EVs can be produced in large quantities and standardized more easily than MSCs, and EVs have a lower risk of immune rejection than MSCs [95][221]. In addition, EVs can deliver therapeutic molecules to target cells, such as microRNAs, which can regulate gene expression and promote neuroprotection [96][222].
6. MSC-EVs in Huntington’s Disease
The progressive loss of brain cells in the putamen, caudate, and cerebral cortex caused by HD, a hereditary neurodegenerative condition, results in physical, mental, and emotional problems. The IT-15 gene has dominant mutations that encourage the development of poly-glutamine (polyQ) repeat sequences in Huntingtin proteins, specifically by boosting the number of CAG repeats inside a polyQ repeat gene sequence. Huntingtin interacts with about 100 other proteins, which suggests that it participates in a variety of biological activities [97][223]. Polyglutamine Huntingtin protein is indeed transported to other cells by the exosome in HD. As a result, exosomes are crucial in the advancement of HD pathogenesis.
Exosomes have been explored as potential treatments for HD [98][99][224,225]. Lee and colleagues conducted research in this area and observed that exosomes derived from adipose-derived mesenchymal stem cells (ADMSC) can regulate harmful characteristics in HD cell models. These exosomes were found to reduce the presence of mHtt intracellular aggregates and increase the expression levels of PGC-1 and phospho-CREB (cAMP response element-binding) [100][226]. Additionally, the same research group investigated the delivery of miR-124 through exosomes to the striatum of R6/2 HD transgenic mice. Despite observing a decrease in the intracellular expression of the miR-124 targeted gene, REST, the effects on the mice’s behavior were minimal [101][227].
Studies have shown that MSC-EVs have particular effects on HD. In vitro analysis has revealed that MSC-EVs can constrain motor function and striatal atrophy in a rat model of HD [102][228]. In their study, Ebrahimi and colleagues showed that the release of GDNF and vascular endothelial growth factor (VEGF) from MSCs had a positive effect on motor coordination and muscle functions in animal models of HD [103][229].
A scalable and dependable technique for loading therapeutic RNA into extracellular vesicles (EVs) has been devised. This method involved incorporating a hydrophobically modified siRNA, designed to target Huntington RNA, into the EVs without causing any adverse effects on their size or structural integrity. The effectiveness of this approach was demonstrated by efficiently silencing Huntington mRNA both in vitro using mouse primary cortical neurons and in vivo after administration into the mouse striatum [104][230].
