Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ahsanullah Unar and Version 2 by Jason Zhu.
Sepsis is a major global health problem that results from a dysregulated and uncontrolled host response to infection, causing organ failure. Despite effective anti-infective therapy and supportive treatments, the mortality rate of sepsis remains high. Approximately 30–80% of patients with sepsis may develop disseminated intravascular coagulation (DIC), which can double the mortality rate. There is currently no definitive treatment approach for sepsis, with etiologic treatment being the cornerstone of therapy for sepsis-associated DIC. Early detection, diagnosis, and treatment are critical factors that impact the prognosis of sepsis-related DIC.
- sepsis
- disseminated intravascular coagulation
- platelets
- mechanism
1. Introduction
Sepsis and disseminated intravascular coagulation (DIC) are interrelated conditions that pose a major threat to global health [1]. In 2001, the International Society for Thrombosis and Hemostasis revised its definition of DIC. DIC is traditionally classified as consumptive coagulopathy, given that its diagnostic criteria are centered around the occurrence of decompensated coagulopathy. However, the definition of DIC also includes the systemic activation of coagulation and endothelial dysfunction, which are integral to its pathophysiology. To reconcile these aspects, the development of disease-specific criteria is underway to enhance both diagnosis and management. In the context of sepsis-associated DIC, innovative strategies, such as a two-step diagnostic process using sepsis-induced coagulopathy (SIC) and the incorporation of new biomarkers, are being considered. As research advances, the need to continually refine understanding of DIC’s specific implications through both laboratory and clinical research remains paramount (Table 1) [2][3][2,3].
Table 1. Review of definitions, pathogenesis, causes, clinical features, and diagnoses of sepsis and associated conditions.
| Condition | Definition | Pathogenesis | Causes | Clinical Features | Diagnosis | Treatment | Reference |
|---|
Table 2. Understanding the molecular mechanisms, clinical manifestations, and therapeutic approaches of sepsis and related conditions.
| Pathology | Molecular Mechanism and Pathways | Clinical Manifestations |
Diagnostic Markers | Therapeutic Approaches |
Relation to Sepsis-3 Controversy | Recent Research Findings | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sepsis | Life-threatening response to infection, causing organ dysfunction. | Dysregulated immune response, leading to inflammation. | Bacterial, viral, fungal infections. | Fever, chills, organ dysfunction. | Clinical symptoms, blood cultures. | Antibiotics, supportive care. | [4] | ||||||||
| Sepsis | Systemic inflammatory response to infection, characterized by PAMPs release and activation of PRRs such as TLRs, triggering NF-κB and MAPK pathways. This results in cytokine storm and potential secondary infections. | Fever, tachycardia, dyspnea, hypotension, altered cognition, organ dysfunction. | Elevated proinflammatory cytokines, leukocytosis, thrombocytopenia, hyperlactatemia, organ dysfunction (SOFA score). | Broad-spectrum antibiotics, fluid resuscitation, vasopressors, corticosteroids, supportive care. | Sepsis-3 emphasizes organ dysfunction, potentially overlooking early sepsis without organ dysfunction. | Focus on early biomarkers and immune response role in sepsis progression. | [4][25][26][4,25,26] | ||||||||
| DIC | Widespread clotting in blood vessels. | Triggered by conditions like infections, trauma. | |||||||||||||
| DIC | Coagulopathy triggered by conditions, including sepsis. Characterized by widespread coagulation activation, microthrombi formation, organ dysfunction, ischemia, and bleeding manifestations. | Sepsis, trauma, malignancies. | Bleeding, thrombosis, organ dysfunction. | Coagulation tests, low platelets. | Address cause, blood transfusions. | Bleeding, purpura, petechiae, organ dysfunction. | Prolonged PT and aPTT, thrombocytopenia, increased FDPs, decreased fibrinogen.[ | Treatment of underlying cause, blood product transfusion, anticoagulants. | Sepsis-3 may not capture DIC complexity in sepsis due to organ dysfunction focus.2 | Exploration of DIC mechanisms in sepsis and potential coagulation cascade modulation.][3][2,3] | |||||
| [ | 3 | ] | [ | 22 | ] | [ | 27][28][29][3,22,27,28,29] | SIC | DIC subset linked to sepsis. | Interaction between inflammation and coagulation. | Prolonged PT, aPTT, decreased platelets. | Organ dysfunction, clot formation. | Focus on organ dysfunction. | ||
| SIC | Anticoagulants. | DIC subset associated with sepsis. Characterized by coagulation activation, fibrinolysis inhibition, clot formation, and potential organ dysfunction. | [ | Similar to DIC, including bleeding, purpura, petechiae, organ dysfunction. | 2 | Prolonged PT and aPTT, thrombocytopenia, increased D-dimer, decreased antithrombin III. | Treatment of underlying sepsis, potential anticoagulants.] | Sepsis-3 may capture SIC patients but may not reflect underlying coagulation abnormalities.[3 | Exploration of SIC mechanisms and potential therapeutic strategies, including anticoagulants.][2, | [3] | |||||
| 30 | ] | [ | 31 | ] | [ | 30 | ,31] | Septic Shock | Severe sepsis subset with high mortality risk. | Acute circulatory failure. | Elevated lactate, decreased platelets. | Hypotension, altered mental state. | Sepsis-3 definition. | Corticosteroids, immunomodulatory drugs. | |
| Septic Shock | Sepsis subset with profound circulatory, cellular, and metabolic abnormalities. Characterized by persistent hypotension unresponsive to fluid resuscitation, requiring vasopressors. | Persistent hypotension, altered cognition, oliguria, tachycardia, dyspnea, cool and clammy skin. | [ | Hyperlactatemia, thrombocytopenia, increased D-dimer, increased procalcitonin, organ dysfunction (SOFA score). | 4 | ] | |||||||||
| Vasopressors, antibiotics, fluid resuscitation, corticosteroids, supportive care. | Sepsis-3 includes septic shock as a subset with increased mortality. Criticized for complexity and need for laboratory results. | Exploration of septic shock pathophysiology and potential therapeutic strategies, including corticosteroids. and immunomodulatory drugs. | [ | 4 | ] | [ | 25][26] | SARS-CoV-2 | Respiratory infection by SARS-CoV-2. | Virus targets ACE2 receptors; can cause ARDS. | SARS-CoV-2 transmission via droplets. | Respiratory symptoms, ARDS. | PCR, chest imaging, serological tests. | Symptomatic relief, antivirals. | [5][6][7][8][5,6,7,8] |
| Flaviviruses | Diseases from viruses like Zika, dengue. | Infection of immune cells, causing imbalanced response. | Mosquito-borne or direct contact. | Fever, rash, potential organ failure. | PCR, serological assays, culturing. | Supportive care, antivirals/antibiotics. | [7][9][10][7,9,10] |
The definition of sepsis has evolved over time, with the latest international consensus defining it as organ failure that results from a dysregulated host response to infection [4]. Although rational antibiotic therapy can control the underlying infection, once triggered, the uncontrolled host response continues to persist, resulting in the high morbidity and mortality observed in sepsis and septic shock (Table 1) [11].
Sepsis is a life-threatening condition that results from a widespread immune-inflammatory response to infection, while DIC is a secondary complication that occurs in up to 80% of patients with sepsis. It is characterized by the systematic activation of the coagulation cascade, leading to the formation of thrombi within the vasculature, with a particular predilection for smaller blood vessels, such as capillaries and organ damage [6]. The interrelation of these conditions highlights the complex nature of bacterial infections and their impact on the body’s immune and clotting systems. Additionally, patients with underlying thrombophilia conditions, characterized by an increased tendency for blood clot formation, are at high risk of developing DIC. This is due to their already primed coagulation system, a status that can be exacerbated by sepsis [12][13][12,13]. This assertion is supported by a body of experimental evidence. For instance, Langerak et al. [14] suggest that individuals with variations in the regulation of their procoagulant, anticoagulant, and fibrinolytic systems, such as those with thrombophilia, may be exposed to additional risk factors for DIC [14]. Furthermore, Prazanowski et al. [15] found that activated protein C resistance (APCR), a genetically determined cause of thrombophilia, can be a risk factor for DIC. Yildirim et al. [16] also suggest that patients with thrombophilia have higher risk scores for conditions such as sepsis-induced coagulopathy (SIC) and DIC. Table 1 provides a comprehensive delineation of the pathology, mechanisms, clinical manifestations, diagnostic markers, and therapeutic interventions associated with SIC, DIC, and related conditions. The correlation of these conditions with the contentious Sepsis-3 definition, in conjunction with the latest research findings, is further elucidated in Table 2. Moreover, Hofstra et al. [17] and Tikkanen et al. [18] both support the notion that thrombophilia can be a risk factor for DIC, with the latter suggesting that the combination of hyperhomocysteinemia and thrombophilia increases the risk of DIC. Therefore, the interrelation of these conditions and the role of thrombophilia as a risk factor for DIC is well supported by the current body of research [14][15][16][17][14,15,16,17]. The mechanisms underlying the relationship between sepsis and DIC are not fully understood. However, researchers have proposed several theories to explain this relationship. One theory suggests that DIC is a direct result of an underlying infection, particularly in cases of IE [19]. The formation of vegetation on heart valves can result in valve dysfunction and the spread of bacteria throughout the bloodstream, leading to sepsis and DIC [20][21][20,21]. Another theory proposes that DIC occurs because of the release of cytokines and other signaling molecules in response to the initial infection. These signaling molecules activate the clotting cascade and lead to the formation of clots [20][22][
| [ | |||||||
| 32 | |||||||
| ] | |||||||
| [ | |||||||
| 4 | , | 25 | , | 26 | , | 32 | ] |
| SARS-CoV-2 | Virus enters host cells via ACE2 receptors, leading to viral replication and immune response activation. In severe cases, cytokine storm leads to severe inflammation and lung tissue damage. | Fever, cough, dyspnea, anosmia, fatigue, organ dysfunction in severe cases. | Positive RT-PCR for SARS-CoV-2, elevated proinflammatory cytokines, abnormal chest imaging. | Antivirals, corticosteroids, monoclonal antibodies, supportive care. | COVID-19 can lead to sepsis-like syndrome. Sepsis-3 may not capture unique aspects of COVID-19-related sepsis. | Focus on understanding severe COVID-19 pathophysiology, immune response role, and potential therapeutic targets. | [5][6][7][8][33][34][35][5,6,7,8,33,34,35] |
| Flaviviruses and Other Microorganisms | Different molecular mechanisms for host cell infection. Flaviviruses infect immune cells, leading to imbalanced immune response. Other microorganisms may produce toxins or virulent factors. | Symptoms vary, may include fever, rash, arthralgia, nausea, vomiting, diarrhea, cough, dyspnea. | Varies, may include positive culture or PCR, elevated proinflammatory cytokines, abnormal imaging. | Varies, may include antibiotics, antivirals, antifungals, supportive care. | Infections can lead to sepsis, but Sepsis-3 may not capture unique aspects of sepsis caused by these pathogens. | Exploration of pathogenesis of sepsis caused by these pathogens and potential therapeutic strategies. | [7][9][10][36][37][38][7,9,10,36,37,38] |
In recent years, researchers have made significant advances in the understanding of sepsis-related DIC, including its causes, diagnosis, and management. For example, the development of new diagnostic tools, such as the use of biomarkers for the early detection of DIC in sepsis, has improved the ability to diagnose and treat sepsis-related DIC [39][40][39,40].
2. Understanding the Role of Bacterial Virulence in the Pathogenesis of Sepsis and Associated Conditions
Researchers have examined the microbiological features of several sepsis-causing bacteria that pose challenges to host defense [41][43]. A group of bacteria, including Staphylococcus aureus, coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Haemophilus influenzae b, Neisseria meningitidis, Klebsiella pneumoniae, Enterococcus faecalis, Acinetobacter baumanii, Escherichia coli, Salmonella enterica, Shigella dysenteriae, Citrobacter freundii, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Bacteroides fragilis, are implicated in severe systemic infections, such as sepsis, DIC, SIC, and septic shock [42][43][44,45]. The controversy surrounding these bacteria lies not in their disease-causing potential but in the mechanisms, they employ and the variability in their pathogenicity. Each bacterium possesses a unique set of virulence factors, and the host’s immune response to these factors can significantly influence the disease outcome [42][43][44,45]. For example, S. aureus produces toxins and biofilms, damages host tissues and immune cells, and provides protection from immune responses and antibiotics [42][44]. CoNS, often overlooked, have emerged as significant pathogens in immunocompromised individuals and those with implanted medical devices [43][45]. S. pneumoniae and H. influenzae b, known for their polysaccharide capsules, evade phagocytosis [44][46]. N. meningitidis can invade the bloodstream, causing sepsis and DIC, with its endotoxin triggering a massive inflammatory response, leading to septic shock [43][45]. Enterobacteriaceae, including K. pneumoniae, E. coli, S. enterica, S. dysenteriae, C. freundii, S. marcescens, and P. mirabilis, possess various virulence factors, causing a range of infections and, in severe cases, sepsis and septic shock [42][43][44,45]. E. faecalis, A. baumanii, P. aeruginosa, and B. fragilis, part of the normal human microbiota, can become opportunistic pathogens, causing infections that can progress to sepsis and septic shock if untreated [42][43][44,45]. The controversy lies in the complex interplay between these bacteria and the host’s immune system [42][44]. The host’s immune response plays a significant role in determining disease outcomes, with an overactive response leading to a ‘cytokine storm’, causing conditions such as sepsis, DIC, and septic shock [41][43][43,45]. Table 3 provides a list of bacteria that cause sepsis and their microbiological features that pose problems to the host defense [41][43].Table 3. Microbiological features of bacteria known to cause sepsis and potentially progress to DIC, SIC, and septic shock, highlighting their impact on host defense.
| Bacteria | GP | CA | SD | HE | SL | CP | SL | BF | RE |
|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | + | + | + | + | + | + | + | + | FAN |
| Coagulase-negative staph | + | + | + | + | + | + | + | + | FAN |
| Streptococcus pneumonia | + | + | + | + | + | + | + | + | FAN |
| Haemophilus influenza b | + | + | + | + | + | + | + | + | MA |
| Neisseria meningitidis | + | + | + | + | + | + | + | + | FAN |
| Klebsiella pneumonia | + | + | + | + | + | + | + | + | FAN |
| Enterococcus faecalis | + | + | + | + | + | + | + | + | FAN |
| Acinetobacter baumanii | + | + | + | + | + | + | + | + | A |
| Escherichia coli | + | + | + | + | + | + | + | + | FAN |
| Salmonella enterica | + | + | + | + | + | + | + | + | FAN |
| Shigella dysenteriae | + | + | + | + | + | + | + | + | FAN |
| Citrobacter freundii | + | + | + | + | + | + | + | + | FAN |
| Serratia marcescens | + | + | + | + | + | + | + | + | FAN |
| Proteus mirabilis | + | + | + | + | + | + | + | + | FAN |
| Pseudomonas aeruginosa | + | + | + | + | + | + | + | + | FAN |
| Bacteroides fragilis | + | + | + | + | + | + | + | + | OAN |
3. Inflammatory Mediators Associated with Pyroptosis and Their Role in Subsequent Coagulation Disorders
Sepsis is a condition caused by infection, with Gram-negative bacterial infections being the most prevalent, accounting for approximately 60% of cases, while Gram-positive bacterial infections account for approximately 40%. When pathogenic microorganisms invade the body, they can be swiftly recognized by the pattern recognition receptors (PRRs) of immune cells, leading to a series of inflammatory responses [45][47]. PRRs are located both on the cell membrane and intracellularly, with Toll-Like Receptor 4 (TLR4) being the most well-studied membrane PRR capable of recognizing lipopolysaccharides (LPS) in Gram-negative bacteria. The inflammasome is a receptor complex that directly detects the presence of pathogenic microorganisms within the cytoplasm. Cytokine storms resulting from excessive activation of the TLR4 receptor by extracellular LPS are the primary cause of DIC. The TLR4 receptors on immune cell membranes, with the aid of coreceptors Myeloid Differentiation Factor 2 (MD2) and Cluster of Differentiation 14 (CD14), recognize highly conserved lipids within extracellular LPS. Signals are conveyed to the cell through Myeloid Differentiation primary response 88 (MyD88), and TIR-domain-containing adapter-inducing interferon-β (TRIF) activates the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB), interferon regulator 3, and other factors, thereby promoting the transcription and secretion of cytokines, chemokines, and other mediators. The activation of the inflammasome thus results in the maturation, release, and pyroptosis of interleukin-1β (IL-1β) and interleukin-18 (IL-18). Pyroptosis is a proinflammatory form of programmed death discovered in recent years that depends on caspases (casp1 and casp 4/5 or casp11 in mice), leading to the cleavage of the N-terminus of the Gasdermin D (GSDMD) protein. Activated GSDMD causes holes in the cell membrane, leading to osmotic swelling, cell death, and the release of large amounts of inflammatory contents, further exacerbating the inflammatory response [46][47][48,49]. Studies have shown that mice with tlr4 knockouts and defects in Casp1 (Casp1−/−) or Casp11 (due to Casp1 chromosome defects) in neighboring encoding genes have demonstrated tolerance to high doses of LPS [48][49][50,51]. Previous research found that Casp1−/− mice from the 129-mouse background were Casp1 and Casp11 double-knockouts, as confirmed by immunoblotting protein analysis. The immunoblotting results indicated that the Casp1 and Casp11 double-knockout mice derived from the 129-background were protein deficient, except for the mice in [50][51][52,53]. A study by Kayagaki’s team found that LPS, with the aid of cholera toxin B (CTB) or certain Gram-negative bacteria, could cause wild-type C57BL/6 mouse BMMs to release IL-1β and trigger pyroptosis, which was not observed in the 129-background mice. C57BL/6 background-derived Casp11−/− mice were found to resist high doses of LPS, while Casp1−/− mice with only the Casp1 deficiency could not resist LPS, indicating that the LPS-induced activation of the Casp11 signaling pathway plays a crucial role in sepsis pathology. Further research revealed that only intracellular LPS can activate Casp11 independently of TLR4 [51][52][53][53,54,55]. Although much research has been performed in recent years on the role of inflammasome activation in sepsis pathology and poor prognosis, the specific mechanism is still not understood. A recent study by Wu et al. elucidated the pivotal role of macrophage pyroptosis in sepsis. During pyroptosis, macrophages undergo cell membrane rupture, leading to the release of tissue factor-containing microparticles (TF MPs). These TF MPs initiate and amplify the extrinsic coagulation cascade, leading to organ failure and DIC, ultimately resulting in the death of the septic host [54][56]. The release of TF MPs from macrophages is contingent on cell lysis, which is dependent on GSDMD. Macrophages deficient in GSDMD do not exhibit the release of TF MPs upon stimulation. Conversely, the release of TF MPs from wild-type macrophages can be inhibited using a cell membrane stabilizer [36]. Wu et al. further substantiated that monocytes/macrophages are the primary sources of TF MPs in sepsis by employing conditional knockout mice and depleting the monocytes/macrophages pharmacologically [54][56]. Pyroptosis of cells, accompanied by the release of substantial amounts of inflammatory mediators, also plays a significant role in subsequent coagulation disorders (Figure 1).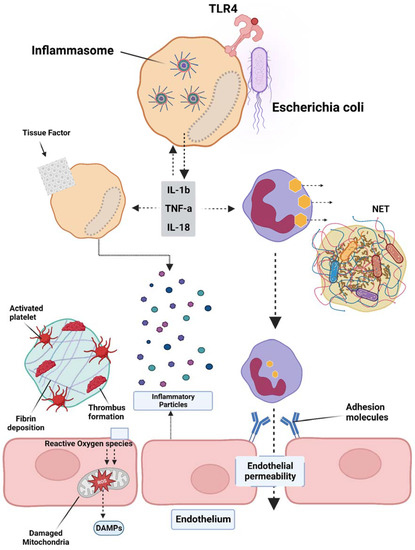
Figure 1. Overview of the pathogenesis of sepsis, which involves several pathophysiological processes, such as endothelial injury, breakdown of the endothelial barrier, immune thrombosis, and disseminated intravascular coagulation (DIC). Various factors contribute to the development of sepsis, including damage-associated molecular patterns (DAMPs), interleukins (ILs), Toll-like receptor 4 (TLR4), and tumor necrosis factor-alpha (TNF-α). The interplay of these factors in the pathogenesis of sepsis leads to the activation of coagulation pathways and inflammatory responses, and organ and organ dysfunction.
4. Platelet Activation and Its Effects on the Coagulation Cascade in Sepsis
TF is a key player in the development of sepsis-related DIC, a pathological condition characterized by abnormal coagulation. Inhibiting TF activity through drugs or gene deletion has been shown to reduce coagulation disorders and decrease mortality in mice with sepsis [55][57]. TF is expressed in various cells surrounding blood vessels, such as pericytes, fibroblasts, and vascular smooth muscle cells, as well as in blood vessels themselves [56][58]. Additionally, activated cells, such as endothelial cells, neutrophils, and eosinophils, were previously believed to express TF; however, further research showed that these cells acquire TF from monocyte-derived MPs through surface receptors [56][58]. The expression of TF on platelets is still a topic of debate, with some studies suggesting that TF can be transferred to the platelet surface after activation and others showing conflicting results. Platelets, in both resting and activated states, have been shown to express varying levels of TF at the mRNA and protein levels; however, this observation has not been consistently supported by all research teams [56][58]. Activated platelets can also be derived through their surface P-selectin or through binding with monocyte MPs or CD15 PSGL-1 (P-Selectin Glycoprotein Ligand-1), which may contribute to the expression of TF on the platelet surface [56][58]. In a study conducted by Pawlinski’s team, a sepsis model was engineered using conditional knockout mice [56][58]. The investigators documented a significant decrease in thrombin-antithrombin complex (TAT) levels 8 h after LPS exposure in mice where tissue factor (TF) was genetically ablated in either hematopoietic or nonhematopoietic cells [56][58]. This pattern was also evident in mice with TF ablation in myeloid cells or in a combination of endothelial and hematopoietic cells. However, the targeted genetic ablation of TF in endothelial cells or vascular smooth muscle cells did not significantly alter the plasma TAT levels in septic mice. These findings underscore the critical role of TF, originating from both myeloid cells and an as-yet-unidentified nonhematopoietic cell source, in instigating the coagulation cascade during sepsis [56][58]. Although the in vitro evidence shows that endothelial cells can express a high level of TF, the in vivo experiments have not consistently found a positive expression of TF on the endothelium. The conditional knockout of TF in endothelial cells also did not have a significant impact on coagulation activation in septic mice. This makes endothelial cells unlikely to be a main source of TF expression or release [57][59]. Wu et al. recently found that by using chlorophosphate liposomes to deplete nearly 90% of the monocytes and macrophages, plasma TAT levels in septic mice were significantly reduced, leading to a >50% increase in survival. This suggests that monocyte/macrophage-derived TF is a major contributor to the activation of sepsis coagulation [54][56]. Previous studies have shown that systemic proinflammatory cytokines resulting from infection cause the overexpression of TF in monocytes/macrophages [58][60]. Numerous animal models of sepsis and clinical studies in sepsis patients have shown a significant increase in the number of circulating TF-positive MPs of monocyte/macrophage origin, which is strongly linked to coagulation activation, organ failure, and death. However, the mechanism behind the formation of these circulating soluble TF MPs has only recently been uncovered. Wu et al. found that proteins from the type III secretory system of bacteria and LPS activate small classical and nonclassical inflammatory components, respectively. Gasdermin D causes macrophage pyroptosis in vivo but does not release TF MPs from cell membrane-bound wells. This process relies on Gasdermin D-mediated osmotic membrane cleavage, which can be significantly reduced with the formation of TF MPs. In sepsis, microvascular damage and the onset of DIC are interrelated. Endothelial cells are critical targets of attack by danger-associated molecular patterns (DAMPs) and inflammatory agents [59][61]. Normally, endothelial cells exert anticoagulant and anti-inflammatory effects; however, upon inflammatory stimulation, exposure to TF in the subendothelial layer triggers exogenous coagulation activation. At the same time, endothelial cells initiate a series of procoagulant and proinflammatory processes by upregulating adhesion molecule expression and attracting and activating immune cells such as monocytes and neutrophils. Secretion of the von Willebrand factor (vWF) also promotes platelet aggregation and platelet-dependent coagulation [59][61]. In sepsis, platelets can be activated by DAMPs, inflammatory mediators, thrombin, and vWF, resulting in increased expression of activated platelet P-selectin, which boosts monocyte TF expression by binding to the PSGL-1 receptors on the surface of monocytes [44][60][46,62]. Activated platelets provide an ample phospholipid surface that significantly amplifies coagulation cascade reactions while reducing blood protease inhibitors, thus inhibiting enzymes in the coagulation reactions [57][59]. Once activated, platelet-dense granules release soluble polyphosphate to their surface, triggering factor XII (FXII) formation and promoting thrombin production via the FXII pathway (Figure 2) [61][63].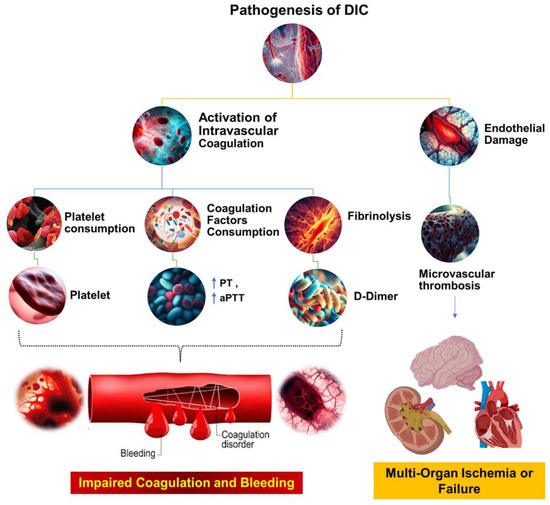
Figure 2. The pathophysiological mechanisms underlying DIC. This figure illustrates the complex cascade of events that occur during the pathogenesis of DIC. The process begins with systemic activation of the coagulation system, which results in extensive fibrin production and deposition. These fibrin deposits then create microvascular thrombi, which can impede blood flow and cause multiorgan dysfunction. Concurrently, significant coagulation activity consumes critical hemostatic components, such as clotting factors and platelets. This depletion can upset the delicate balance of hemostasis, potentially leading to severe, life-threatening bleeding. Prothrombin time (PT); aPTT (activated partial thromboplastin time).
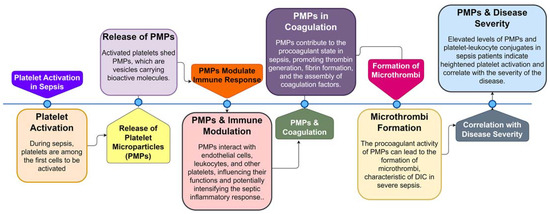
Figure 3. Sequential Role of Platelets and Platelet Microparticles (PMPs) in Sepsis Pathogenesis. This flowchart illustrates the step-by-step involvement of platelets and PMPs during sepsis, from initial platelet activation to the formation of microthrombi and the correlation of PMP levels with disease severity.
