Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Junming Wang.
Chronic inflammatory respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis, present challenges in terms of effective treatment and management. These diseases are characterized by persistent inflammation in the airways, leading to structural changes and compromised lung function. To achieve optimal therapeutic outcomes while minimizing systemic side effects, targeted therapies and precise drug delivery systems are crucial to the management of these diseases.
- drug delivery
- chronic inflammatory respiratory diseases
- nanoparticle-based drug delivery systems
- inhaled corticosteroids
1. Introduction
Chronic inflammatory respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), affect millions of people worldwide and are a leading cause for the increase in lung disease morbidity and mortality [1]. Asthma, as a heterogeneous clinical syndrome, affects over 300 million people worldwide [2]. COPD, a disease mainly associated with long-term smoking, became the third leading cause of death globally in 2020 [3]. Although there are several existing treatments, limited efficacy, side effects, and individual variability still cannot be ignored [4,5,6][4][5][6]. In recent years, there has been a growing interest in the development of targeted drug delivery systems for the treatment of these diseases [7,8,9][7][8][9]. Nanoparticle-based drug delivery systems, inhaled corticosteroids (ICSs), novel biologicals, gene therapy, and personalized medicine have emerged as promising approaches to deliver drugs more effectively and with fewer side effects.
Currently, the development of new nanoparticle-based drug delivery systems that can target specific cells such as lung epithelial cells and macrophages, while minimizing systemic side effects, have received significant attention [10]. These systems utilize nanoparticles, which are tiny particles ranging from 1 to 100 nanometers in size, to encapsulate and deliver drugs directly to the affected areas of the lungs [11]. By modifying the surface properties of nanoparticles, researchers can enhance their ability to selectively bind to specific cell types in the lungs, thereby improving drug delivery efficiency and reducing off-target effects [12]. Furthermore, nanoparticle-based drug delivery systems can protect the drugs from degradation and enhance their stability, ensuring sustained release and prolonged therapeutic effects [13].
In addition to nanoparticle-based systems, inhaled corticosteroids (ICSs) have long been used as a standard treatment for chronic inflammatory respiratory diseases [14,15][14][15]. ICSs work by reducing inflammation in the airways, thus alleviating symptoms and preventing exacerbation. Researchers are also exploring novel biological targets and innovative methods for delivering biologicals to the lungs. Gene therapy approaches, including viral-vector-based delivery systems and CRISPR–Cas9 technology, represent another exciting frontier in the treatment of chronic inflammatory respiratory diseases [16,17][16][17]. Moreover, personalized medicine approaches take into account an individual’s unique characteristics, such as genetics, biomarkers, and lifestyle factors, to tailor treatments to their specific needs [8,18][8][18]. By utilizing advanced diagnostic tools like genomic sequencing and biomarker analysis, healthcare providers can identify patient subgroups who are more likely to respond to a particular therapy, thus optimizing treatment outcomes [19,20][19][20]. However, several challenges remain, including optimizing delivery efficiency, ensuring safety, and addressing ethical considerations.
2. Nanoparticle-Based Drug Delivery Systems
The application of nanotechnology continues to provide effective strategies in treating various chronic diseases and improving treatment outcomes. Using nanocarrier systems such as liposomes, micelles, and nanoparticles for pulmonary drug delivery has been proven to be a promising noninvasive treatment strategy for achieving drug deposition and controlled release in the lungs [10] (Figure 1). These systems involve the use of engineered particles with dimensions on the nanometer scale to deliver drugs directly to target cells in the lungs [21]. Nanoparticles have several advantages over conventional drug delivery methods, including improved bioavailability, enhanced targeting, and reduced toxicity [22,23][22][23].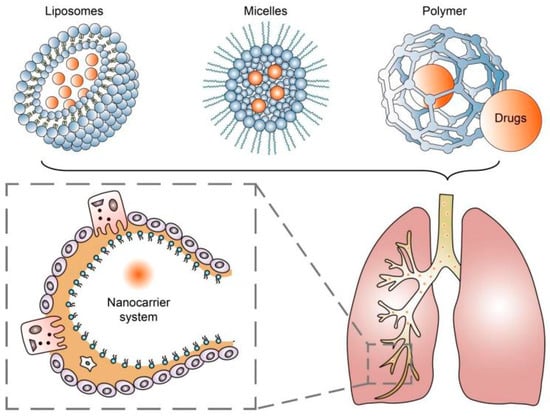
Figure 1.
Nanocarrier systems can achieve drug deposition and controlled release in the lungs.
Table 1.
Therapeutic applications of nanoparticles in chronic inflammatory respiratory diseases.
| Diseases | Type of Nanoparticles | Drugs | Target Ligands | Targets | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asthma | SPION | None | IL4Rα monoclonal antibody | ASMs | [35] | ||||||
| SPION | None | Anti-ST2 blocking antibodies | Inflammatory lung tissue | [36] | |||||||
| PLGA-based nanoparticles | Smart silencer of Dnmt3aos | Exosome membrane of M2 macrophages | M2 macrophages | [40] | |||||||
| [ | 78 | ] | LNP | Polyinosinic-polycytidylic acid | None | Lung epithelial cells |
[41] | ||||
| Dry powder inhalers (DPIs) | Single- and multi-unit doses | Deliver medication directly to the lungs in a powdered form. | Breath-activated, portable, and do not require coordination between inhalation and device activation. | Require adequate inspiratory flow for optimal drug delivery, and can be used only with specific types of dry powder medications. | [79] | COPD | HNP | siRNA against SCNN1A and SCNN1B | None | Lung epithelial cells |
[42] |
| LNP | siRNA against TNF-α | None | None | [43] | |||||||
| IPF | LNP | siRNA against IL-11 | None | MLFs | [44] | ||||||
| CF | LNP | Plasmid DNA | ICAM-1 targeting peptide |
Lung epithelial cells |
[45] |
Abbreviations: SPION: Superparamagnetic iron oxide nanoparticle; IL4Rα: Interleukin-4 receptor alpha; ASM: Airway smooth muscle cell; ST2: Grow stimulation expressed gene 2; PLGA: Polylactic-co-glycolic acid; LNP: Lipid nanoparticle; HNP: Hybrid nanoparticle; SCNN1A: Sodium channel non-alpha subunit 1A; SCNN1B: Sodium channel non-alpha subunit 1B; TNF-α: Tumor necrosis factor alpha; IL-11: Interleukin-11; MLFs: Mouse lymphatic fibroblasts; ICAM-1: Intercellular adhesion molecule-1.
Despite the promise of nanoparticle-based drug delivery, there are still several research challenges that need to be addressed. For example, there is a need to develop nanoparticles with optimal physicochemical properties, such as particle size, surface charge, and stability, to ensure effective drug delivery [46]. Recent research has reported that the structure of mesoporous silica nanoparticles (MSNs) can be well controlled with several parameters such as pH, surfactant, silica precursor, and temperature. For instance, Pan et al. prepared a series of size-controlled MSNs with a range of 25–105 nm by simply changing the amount of the basic catalyst triethanolamine (TEA) added [47]. So, it is believed that MSNs have significant potential to serve as nanocarriers for pulmonary drug delivery [48]. Additionally, researchers need to carefully evaluate the safety and toxicity of nanoparticle-based drug delivery systems. While some studies have shown promising results, others have raised concerns about the potential for long-term toxicity and negative environmental impacts of nanoparticle-based drug delivery [49,50][49][50]. Currently, it is widely believed that the cytotoxicity of nanoparticles is mainly related to their large surface area and small size [51]. Yuan et al. concluded through their study on the effects of 20, 30, and 40 nm zinc oxide nanoparticles on human embryonic lung fibroblasts that cytotoxicity is concentration-dependent, therefore calling for the minimum therapeutic concentration [52]. Other researchers found that the surface charge and solubility are also associated with the cytotoxicity of nanoparticles [53,54][53][54].
Moving forward, researchers are exploring several future directions for nanoparticle-based drug delivery systems. For example, considering that there is a large amount of mucus oozing out of the lungs during chronic inflammatory diseases, researchers are developing new mucus-penetrating nanoparticles (MPPs). Uptake mechanism studies revealed that caveolae-mediated endocytosis and macropinocytosis contributed to the absorption of MPPs [55]. In vivo research results showed a more than five-fold increase in drug bioavailability [56]. Others are investigating new methods for optimizing nanoparticle design and surface modification to improve targeting and drug release [40,57][40][57]. Additionally, some researchers are investigating the potential of combining nanoparticles with other treatment modalities such as gene therapy or immunotherapy [46,58][46][58]. Finally, there is growing interest in developing personalized nanoparticle-based drug delivery approaches that can be tailored to individual patients based on their unique disease characteristics and genetic profiles [59].
Through targeted drug delivery, nanoparticles have the potential to improve therapeutic efficacy and reduce systemic side effects. Overall, nanoparticle-based drug delivery systems hold great promise for the treatment of chronic inflammatory respiratory diseases.
While there have been notable advancements, it is important to acknowledge that there are still existing limitations concerning the use of ICSs that necessitate careful consideration and remediation. For example, some studies have suggested that long-term use of ICSs may increase the risk of pneumonia and cataracts [82,83][82][83]. Moreover, further research is needed to determine the optimal ICS dose and duration of treatment for individual patients [84].
Future directions for research in ICS delivery are focused on several areas. Personalized ICS dosing strategies based on individual patient characteristics and disease severity are being explored [85]. Investigations are currently underway to explore new ICS formulations that utilize innovative drug delivery technologies, including nanotechnology and microencapsulation [86].
Thus, ICSs remain an effective treatment option for chronic respiratory diseases, but proper delivery optimization is crucial to their efficacy and safety.
3. Inhaled Corticosteroids (ICSs)
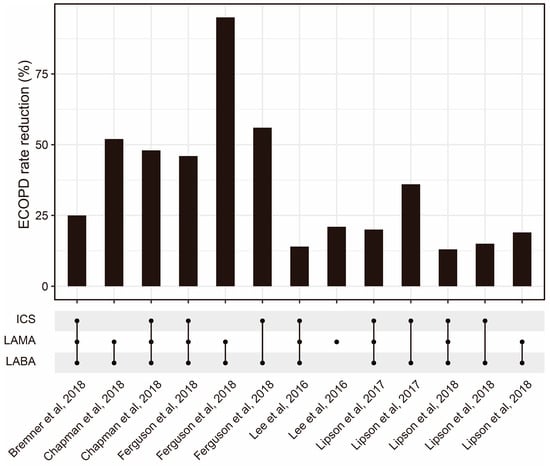
Inhaled corticosteroids (ICSs) are widely used as a treatment option for chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). These medications work by reducing the production of inflammatory mediators in the airways, which helps prevent or reduce inflammation, bronchoconstriction, and mucus production. According to the Global Initiative for Asthma (GINA) report [1], ICSs have been shown to improve lung function, reduce exacerbation, and improve quality of life in patients with chronic respiratory diseases. However, there are some current challenges with ICS delivery that limit their efficacy. One major challenge is achieving the optimal distribution of the medication throughout the lungs. ICS particles can become trapped in the mouth or throat, reducing their effectiveness in the lower airways [60]. Patients may also have difficulty using their inhaler correctly, leading to reduced medication delivery and efficacy [61]. Moreover, selecting the appropriate ICS dose for each patient can be challenging, as individual needs can vary significantly [62]. To optimize ICS delivery and improve its efficacy, several methods have been developed. One approach involves the use of spacer devices, which help to slow down the speed of medication delivery and improve medication deposition in the lungs [63]. Another approach is the development of more efficient ICS formulations, such as fine-particle ICSs, which have shown improved efficacy compared with conventional ICS formulations [64]. Fine-particle ICSs have greater deposition in the small airways compared with conventional ICSs [65]. According to a meta-analysis, fine-particle ICSs have significantly higher odds of achieving asthma control [66]. The combination of ICSs and other drugs is also worth further optimization (Figure 2). Additionally, research advancements have explored smart inhalers that can monitor medication adherence and provide feedback to patients [67]. Nowadays, four kinds of inhalers (nebulizers, dry powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft mist inhalers (SMIs)) are widely used (Table 2). Recently, artificial intelligence (AI)-based intelligent inhalers have attracted much attention, as they can enable real-time regulation of inhalation plans. For example, intelligent dry powder inhalers (DPIs) constructed based on artificial neural networks (ANNs) have effectively improved the bioavailability of drugs [68], but additional data are still needed to train more advanced models to output better drug delivery plans [69].
Figure 2. The ECOPD rate reduction from ICSs combined with other drug regimens reported by some published studies [70,71,72,73,74,75][70][71][72][73][74][75]. Abbreviations: ECOPD: Exacerbation of chronic obstructive pulmonary disease; ICS: Inhaled corticosteroid; LAMA: Long-acting muscarinic antagonist; LABA: Long-acting beta2-adrenergic agonist.
Table 2.
Different kinds of ICS inhalers.
| Type of Inhaler | Subtype | Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Nebulizers | Jet (or pneumatic) | Use compressed air or oxygen to convert liquid medication into a fine mist for inhalation. | Versatile and suitable for all ages. | Longer administration times, produce noise and vibration, require power sources, and need regular maintenance. | [76] |
| Ultrasonic | Use high-frequency vibrations to convert liquid medication into a fine mist for inhalation. | Portable and compact, have faster administration times, operate quietly. | Not suitable for medications that are heat-sensitive or contain suspensions. | [77] | |
| Mesh | Use a vibrating mesh or perforated plate to generate a fine aerosol mist from liquid medication. | Portable, lightweight, and operate silently with faster administration times. | Have limitations in delivering higher viscosity medications or large medication volumes. | ||
| Pressurized metered-dose inhalers (pMDIs) | Single and combined drugs | Deliver medication in a pressurized aerosol form using propellants. | Deliver a consistent dose, require minimal preparation time. | The presence of propellants and the inability to assess remaining medication levels easily. | [80] |
| Soft mist inhalers (SMIs) | None | Deliver medication as a slow-moving aerosol mist. | Provide consistent and precise dosing, generate a slow-moving mist suitable for patients with diverse inspiratory abilities, and are equipped with dose counters to monitor medication levels. | Potential clogging if not used properly, higher cost compared with other inhalers, and limited availability of medications in soft mist formulation. | [81] |
References
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2021, 59, 2102730.
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15.
- Raherison, C.; Girodet, P.-O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221.
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in asthma: New understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441.
- Christenson, S.A.; Smith, S.A.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242.
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074.
- Porsbjerg, C.M.; Sverrild, A.; Lloyd, C.M.; Menzies-Gow, A.N.; Bel, E.H. Anti-alarmins in asthma: Targeting the airway epithelium with next-generation biologics. Eur. Respir. J. 2020, 56, 2000260.
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651.
- Wang, J.; Hu, K.; Cai, X.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin. B 2022, 12, 18–32.
- Forest, V.; Pourchez, J. Nano-delivery to the lung—By inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173.
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96.
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2021, 18, 595–606.
- Oh, J.Y.; Yang, G.; Choi, E.; Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 10, 1448–1455.
- Heo, Y.-A. Budesonide/Glycopyrronium/Formoterol: A Review in COPD. Drugs 2021, 81, 1411–1422.
- Cloutier, M.M.; Dixon, A.E.; Krishnan, J.A.; Lemanske, R.F.; Pace, W.; Schatz, M. Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update from the National Asthma Education and Prevention Program. JAMA 2020, 324, 2301–2317.
- Werder, R.B.; Liu, T.; Abo, K.M.; Lindstrom-Vautrin, J.; Villacorta-Martin, C.; Huang, J.; Hinds, A.; Boyer, N.; Bullitt, E.; Liesa, M.; et al. CRISPR interference interrogation of COPD GWAS genes reveals the functional significance of desmoplakin in iPSC-derived alveolar epithelial cells. Sci. Adv. 2022, 8, eabo6566.
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53.
- Wouters, E.F.; Wouters, B.B.; Augustin, I.M.; Houben-Wilke, S.; Vanfleteren, L.E.; Franssen, F.M. Personalised pulmonary rehabilitation in COPD. Eur. Respir. Rev. 2018, 27, 170125.
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12.
- Castaldi, P.J.; Yun, J.; Estepar, R.S.J.; Ross, J.C.; Cho, M.H.; Hersh, C.P.; Kinney, G.L.; Young, K.A.; Regan, E.A.; Lynch, D.A.; et al. Machine Learning Characterization of COPD Subtypes. Chest 2020, 157, 1147–1157.
- Passi, M.; Shahid, S.; Chockalingam, S.; Sundar, I.K.; Packirisamy, G. Conventional and Nanotechnology Based Approaches to Combat Chronic Obstructive Pulmonary Disease: Implications for Chronic Airway Diseases. Int. J. Nanomed. 2020, 15, 3803–3826.
- Yhee, J.Y.; Im, J.; Nho, R.S. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J. Clin. Med. 2016, 5, 82.
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718.
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757.
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571.
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185.
- Pohlit, H.; Bellinghausen, I.; Frey, H.; Saloga, J. Recent advances in the use of nanoparticles for allergen-specific immunotherapy. Allergy 2017, 72, 1461–1474.
- Kularatne, S.A.; Low, P.S. Targeting of Nanoparticles: Folate Receptor. Cancer Nanotechnol. 2010, 624, 249–265.
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851.
- Xu, H.; Yao, Q.; Cai, C.; Gou, J.; Zhang, Y.; Zhong, H.; Tang, X. Amphiphilic poly(amino acid) based micelles applied to drug delivery: The in vitro and in vivo challenges and the corresponding potential strategies. J. Control. Release 2015, 199, 84–97.
- Sahib, M.; Darwis, Y.; Peh, K.K.; Abdulameer, S.; Tan, Y.T.F. Rehydrated sterically stabilized phospholipid nanomicelles of budesonide for nebulization: Physicochemical characterization and in vitro, in vivo evaluations. Int. J. Nanomed. 2011, 6, 2351–2366.
- Zhang, T.; Xu, Q.; Huang, T.; Ling, D.; Gao, J. New Insights into Biocompatible Iron Oxide Nanoparticles: A Potential Booster of Gene Delivery to Stem Cells. Small 2020, 16, e2001588.
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 1–29.
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113.
- Halwani, R.; Shaik, A.S.; Ratemi, E.; Afzal, S.; Kenana, R.; Al-Muhsen, S.; Al Faraj, A. A novel anti-IL4Rα nanoparticle efficiently controls lung inflammation during asthma. Exp. Mol. Med. 2016, 48, e262.
- Wu, Y.; Shi, W.; Wang, H.; Yue, J.; Mao, Y.; Zhou, W.; Kong, X.; Guo, Q.; Zhang, L.; Xu, P.; et al. Anti-ST2 Nanoparticle Alleviates Lung Inflammation by Targeting ILC2s-CD4+T Response. Int. J. Nanomed. 2020, 15, 9745–9758.
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320.
- Zuo, X.; Guo, X.; Gu, Y.; Zheng, H.; Zhou, Z.; Wang, X.; Jiang, S.; Wang, G.; Xu, C.; Wang, F. Recent Advances in Nanomaterials for Asthma Treatment. Int. J. Mol. Sci. 2022, 23, 14427.
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695.
- Pei, W.; Li, X.; Bi, R.; Zhang, X.; Zhong, M.; Yang, H.; Zhang, Y.; Lv, K. Exosome membrane-modified M2 macrophages targeted nanomedicine: Treatment for allergic asthma. J. Control. Release 2021, 338, 253–267.
- Dauletbaev, N.; Cammisano, M.; Herscovitch, K.; Lands, L.C. Stimulation of the RIG-I/MAVS Pathway by Polyinosinic:Polycytidylic Acid Upregulates IFN-β in Airway Epithelial Cells with Minimal Costimulation of IL-8. J. Immunol. 2015, 195, 2829–2841.
- D’Angelo, I.; Costabile, G.; Durantie, E.; Brocca, P.; Rondelli, V.; Russo, A.; Russo, G.; Miro, A.; Quaglia, F.; Petri-Fink, A.; et al. Hybrid Lipid/Polymer Nanoparticles for Pulmonary Delivery of siRNA: Development and Fate Upon In Vitro Deposition on the Human Epithelial Airway Barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 170–181.
- Dormenval, C.; Lokras, A.; Cano-Garcia, G.; Wadhwa, A.; Thanki, K.; Rose, F.; Thakur, A.; Franzyk, H.; Foged, C. Identification of Factors of Importance for Spray Drying of Small Interfering RNA-Loaded Lipidoid-Polymer Hybrid Nanoparticles for Inhalation. Pharm. Res. 2019, 36, 142.
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.-Q. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 2022, 8, eabn7162.
- Tagalakis, A.D.; McAnulty, R.J.; Devaney, J.; Bottoms, S.E.; Wong, J.B.; Elbs, M.; Writer, M.J.; Hailes, H.C.; Tabor, A.B.; O’Callaghan, C.; et al. A Receptor-targeted Nanocomplex Vector System Optimized for Respiratory Gene Transfer. Mol. Ther. 2008, 16, 907–915.
- Blank, F.; Fytianos, K.; Seydoux, E.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; von Garnier, C.; Rothen-Rutishauser, B. Interaction of biomedical nanoparticles with the pulmonary immune system. J. Nanobiotechnol. 2017, 15, 6.
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725.
- García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953.
- Kim, D.E.; Lee, Y.; Kim, M.; Lee, S.; Jon, S.; Lee, S.-H. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials 2017, 140, 37–44.
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.; Sohn, J. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271.
- Ali, M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv. Colloid Interface Sci. 2023, 314, 102881.
- Yuan, J.-H.; Chen, Y.; Zha, H.-X.; Song, L.-J.; Li, C.-Y.; Li, J.-Q.; Xia, X.-H. Determination, characterization and cytotoxicity on HELF cells of ZnO nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 145–150.
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374.
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334.
- Cheng, H.; Cui, Z.; Guo, S.; Zhang, X.; Huo, Y.; Mao, S. Mucoadhesive versus mucopenetrating nanoparticles for oral delivery of insulin. Acta Biomater. 2021, 135, 506–519.
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89.
- da Silva, A.L.; de Oliveira, G.P.; Kim, N.; Cruz, F.F.; Kitoko, J.Z.; Blanco, N.G.; Martini, S.V.; Hanes, J.; Rocco, P.R.M.; Suk, J.S.; et al. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci. Adv. 2020, 6, eaay7973.
- Hou, C.; Bai, H.; Wang, Z.; Qiu, Y.; Kong, L.-L.; Sun, F.; Wang, D.; Yin, H.; Zhang, X.; Mu, H.; et al. A hyaluronan-based nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohydr. Polym. 2018, 195, 339–348.
- Kubczak, M.; Michlewska, S.; Bryszewska, M.; Aigner, A.; Ionov, M. Nanoparticles for local delivery of siRNA in lung therapy. Adv. Drug Deliv. Rev. 2021, 179, 114038.
- Latorre, M.; Bacci, E.; Seccia, V.; Bartoli, M.L.; Cardini, C.; Cianchetti, S.; Cristofani, L.; Di Franco, A.; Miccoli, M.; Puxeddu, I.; et al. Upper and lower airway inflammation in severe asthmatics: A guide for a precision biologic treatment. Ther. Adv. Respir. Dis. 2020, 14, 1753466620965151.
- Aalbers, R.; Vogelmeier, C.; Kuna, P. Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respir. Med. 2016, 111, 1–7.
- Evans, D.J.; Kew, K.M.; Anderson, D.E.; Boyter, A.C. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database Syst. Rev. 2015, 2015, CD011437.
- Dorinsky, P.; DePetrillo, P.; DeAngelis, K.; Trivedi, R.; Darken, P.; Gillen, M. Relative Bioavailability of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Administered with and without a Spacer: Results of a Phase I, Randomized, Crossover Trial in Healthy Adults. Clin. Ther. 2020, 42, 634–648.
- Guilbert, T.W.; Colice, G.; Grigg, J.; van Aalderen, W.; Martin, R.J.; Israel, E.; Postma, D.S.; Roche, N.; Phipatanakul, W.; Hillyer, E.V.; et al. Real-Life Outcomes for Patients with Asthma Prescribed Spacers for Use with Either Extrafine- or Fine-Particle Inhaled Corticosteroids. J. Allergy Clin. Immunol. Pract. 2017, 5, 1040–1049.e4.
- Postma, D.S.; Dekhuijzen, R.; van der Molen, T.; Martin, R.J.; van Aalderen, W.; Roche, N.; Guilbert, T.W.; Israel, E.; van Eickels, D.; Khalid, J.M.; et al. Asthma-Related Outcomes in Patients Initiating Extrafine Ciclesonide or Fine-Particle Inhaled Corticosteroids. Allergy Asthma Immunol. Res. 2017, 9, 116–125.
- Sonnappa, S.; McQueen, B.; Postma, D.S.; Martin, R.J.; Roche, N.; Grigg, J.; Guilbert, T.; Gouder, C.; Pizzichini, E.; Niimi, A.; et al. Extrafine Versus Fine Inhaled Corticosteroids in Relation to Asthma Control: A Systematic Review and Meta-Analysis of Observational Real-Life Studies. J. Allergy Clin. Immunol. Pract. 2018, 6, 907–915.e7.
- Chan, A.; De Simoni, A.; Wileman, V.; Holliday, L.; Newby, C.J.; Chisari, C.; Ali, S.; Zhu, N.; Padakanti, P.; Pinprachanan, V.; et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Syst. Rev. 2022, 6, CD013030.
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2016, 14, 499–512.
- Gaikwad, S.S.; Pathare, S.R.; More, M.A.; Waykhinde, N.A.; Laddha, U.D.; Salunkhe, K.S.; Kshirsagar, S.J.; Patil, S.S.; Ramteke, K.H. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J. Control. Release 2023, 355, 292–311.
- Bremner, P.R.; Birk, R.; Brealey, N.; Ismaila, A.S.; Zhu, C.Q.; Lipson, D.A. Single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: A randomized non-inferiority study. Respir. Res. 2018, 19, 19.
- Chapman, K.R.; Hurst, J.R.; Frent, S.-M.; Larbig, M.; Fogel, R.; Guerin, T.; Banerji, D.; Patalano, F.; Goyal, P.; Pfister, P.; et al. Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 329–339.
- Ferguson, G.T.; Rabe, K.F.; Martinez, F.J.; Fabbri, L.M.; Wang, C.; Ichinose, M.; Bourne, E.; Ballal, S.; Darken, P.; DeAngelis, K.; et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir. Med. 2018, 6, 747–758.
- Lee, S.-D.; Xie, C.-M.; Yunus, F.; Itoh, Y.; Ling, X.; Yu, W.-C.; Kiatboonsri, S. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: A randomized, multicentre study in East Asia. Respirology 2016, 21, 119–127.
- Lipson, D.A.; Barnacle, H.; Birk, R.; Brealey, N.; Locantore, N.; Lomas, D.A.; Ludwig-Sengpiel, A.; Mohindra, R.; Tabberer, M.; Zhu, C.-Q.; et al. FULFIL Trial: Once-Daily Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 438–446.
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680.
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900.
- Khan, I.; Hussein, S.; Houacine, C.; Sadozai, S.K.; Islam, Y.; Bnyan, R.; Elhissi, A.; Yousaf, S. Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. Int. J. Pharm. 2021, 598, 120376.
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958.
- Montoro, J.; Antolín-Amérigo, D.; Izquierdo-Domínguez, A.; Zapata, J.; González, G.; Valero, A. Impact of Asthma Inhalers on Global Climate: A Systematic Review of Their Carbon Footprint and Clinical Outcomes in Spain. J. Investig. Allergol. Clin. Immunol. 2023, 33, 250–262.
- Kaur, I.; Aggarwal, B.; Gogtay, J. Integration of dose counters in pressurized metered-dose inhalers for patients with asthma and chronic obstructive pulmonary disease: Review of evidence. Expert Opin. Drug Deliv. 2015, 12, 1301–1310.
- Komalla, V.; Wong, C.Y.J.; Sibum, I.; Muellinger, B.; Nijdam, W.; Chaugule, V.; Soria, J.; Ong, H.X.; Buchmann, N.A.; Traini, D. Advances in soft mist inhalers. Expert Opin. Drug Deliv. 2023.
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219.
- Mattishent, K.; Thavarajah, M.; Blanco, P.; Gilbert, D.; Wilson, A.M.; Loke, Y.K. Meta-Review: Adverse Effects of Inhaled Corticosteroids Relevant to Older Patients. Drugs 2014, 74, 539–547.
- Calzetta, L.; Cazzola, M.; Matera, M.G.; Rogliani, P. Adding a LAMA to ICS/LABA Therapy: A Meta-analysis of Triple Combination Therapy in COPD. Chest 2019, 155, 758–770.
- Fitzpatrick, A.M.; Jackson, D.J.; Mauger, D.T.; Boehmer, S.J.; Phipatanakul, W.; Sheehan, W.J.; Moy, J.N.; Paul, I.M.; Bacharier, L.B.; Cabana, M.D.; et al. Individualized therapy for persistent asthma in young children. J. Allergy Clin. Immunol. 2016, 138, 1608–1618.e12.
- Casula, L.; Sinico, C.; Valenti, D.; Pini, E.; Pireddu, R.; Schlich, M.; Lai, F.; Fadda, A.M. Delivery of beclomethasone dipropionate nanosuspensions with an electronic cigarette. Int. J. Pharm. 2021, 596, 120293.
More
