Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Hoda Moazzen.
Desmosomes play a vital role in providing structural integrity to tissues that experience significant mechanical tension, including the heart. Deficiencies in desmosomal proteins lead to the development of arrhythmogenic cardiomyopathy (AC). The limited availability of preventative measures in clinical settings underscores the pressing need to gain a comprehensive understanding of desmosomal proteins not only in cardiomyocytes but also in non-myocyte residents of the heart, as they actively contribute to the progression of cardiomyopathy.
- desmosome
- cardiogenesis
- cell fate determination
1. Cardiogenesis and Mechanical Cues
Cardiogenesis is the orchestrated act of cell proliferation, differentiation, and migration that results in the formation of a resilient and durable contractile organ. The uninterrupted rhythmic contractions and the specific arrangement of cardiomyocytes and non-cardiomyocytes are the essential pillars of cardiac function. Deviation from cardiac cell identity and the canonical cell arrangement therefore not only alters cardiac morphogenesis but also impairs heart function. OurThe knowledge about the key genes that drive cardiogenesis comes from decades of animal studies [1]. It is increasingly recognized that the genetic profile and signaling molecules are not the only regulators of cardiac cell biology, but that mechanical cues also play a definitive role [2]. For example, the stiffness of the extracellular matrix modulates signaling in cardiomyocytes during development and disease [3][4]. Such multifactorial pathogenesis has been discussed in various cardiomyopathies including the still poorly understood genetic heart disease arrhythmogenic cardiomyopathy (AC).
Fifty percent of AC cases have been linked to mutations in genes that contribute to the formation of desmosomes, which are prominent cell–cell adhesion sites between cardiomyocytes [5][6]. Disease hallmarks are arrhythmia, loss of cardiomyocytes, and progressive formation of fibro–fatty structures, which lead to impairment of heart function and ultimately to heart failure [7]. Several transgenic animal models have been created to study AC pathogenesis [6]. The emerging insights categorize AC as a multifaceted disease involving disruption of mechanical and signaling pathways and a surge of immune responses [8]. This preseaperrch does not intend to provide a comprehensive review of AC pathogenesis and its clinical outcomes. Readers are referred to recent excellent reviews [9][10][11][12]. Instead, wresearchers will focus on the much less investigated and poorly understood functions of desmosomes in embryogenesis.
Desmosomal proteins are indispensable for early embryogenesis [13][14][15] and cardiogenesis [16][17]. Desmosomal proteins are enriched in intercalated discs of cardiomyocytes, but have been detected at low levels also in non-cardiomyocytes such as epicardial cells [18][19][20] and cardiac mesenchymal cells [21][22][23]. Importantly, cardiac mesenchymal cells also express desmosomal proteins [24] and desmosomal protein deficiency can enhance differentiation of these cells into fibrous or adipose tissue. In a recent study, a role of desmosomes has been suggested in regulation of cardiac mesenchymal cell fate by direct modulation of Ca2+ signaling at the level of gene expression [25].
During the last decades, research in the AC field was dominated by investigating the role of desmosomal proteins in adult cardiomyocytes [8][26]. It, however, remains to be explored whether desmosomal deficiency triggers “primary events” for the renewal or differentiation of cardiac progenitors both in the developing and mature heart. Of note, molecular pathways that are activated during embryonic development can be reactivated in pathological conditions [27].
2. Molecular Structure of Desmosomes
Desmosomes are specialized structures, which support the physical stability and integrity of epithelial and heart tissue. Cardiac desmosomes are formed by clustering of the Ca2+-dependent adhesion molecules (cadherins) desmoglein 2 (Dsg2) and desmocollin 2 (Dsc2) in the plasma membrane. They interact with each other via their extracellular domains to link neighboring cells. Intracellularly, the clustered desmosomal cadherins are connected to plakophilin 2 (Pkp2) and plakoglobin (PG), both of which contain multiple copies of the 42 amino acid-long armadillo repeat. They serve not only as structural linkers in desmosomes but fulfill additional cellular functions impacting adherens junctions, cytoskeletal organization, and gene transcription. The functions of the large cytolinker desmoplakin (Dsp), on the other hand, are much more restricted to desmosomes. Dsp is essential for the linkage between the clustered desmosomal cadherins with their associated armadillo-repeat proteins and the intermediate filament cytoskeleton, which consists of desmin polypeptides in cardiomyocytes (Figure 1A) [28][29]. Although the molecular composition of desmosomes is the same in the embryonic and adult heart, the arrangement of desmosomes differs. A distinctive characteristic of mammalian adult cardiomyocytes is their maturation process, which is initiated postnatally. Round-shaped embryonic cardiomyocytes initially form independent adherens junctions and desmosomes with neighboring cells throughout their entire borders. Concurrent with myofibril elongation and organization, desmosomes and adherens junctions concentrate at the apical surfaces of cardiomyocytes where they serve important mechanical functions [30]. Postnatally, the junctions amalgamate forming hybrid junctions that are composed of tightly integrated desmosomes and adherens junctions, which are in close apposition to gap junctions and membrane channels. This supercomplex has been referred to as area composita and connexome [31][32]. It was suggested that the maturation phase is essential to ensure life-long contraction of cardiomyocytes [31][33].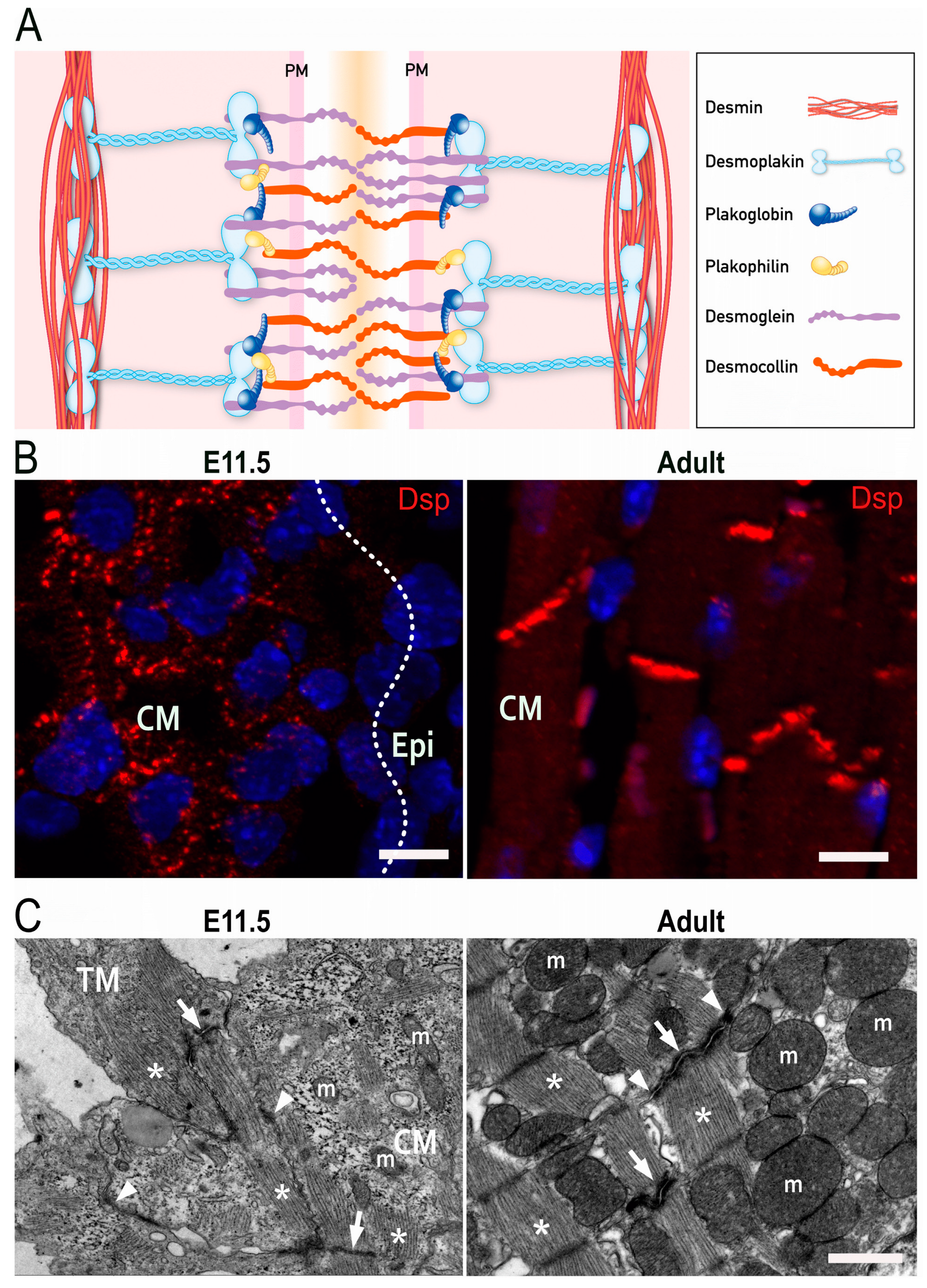
Figure 1. Desmosomes in the heart. (A) Scheme of the molecular desmosome structure. PM: plasma membrane. (B) Fluorescence microscopy detecting desmoplakin (Dsp; red) and nuclei (blue) in embryonic (left) and adult murine heart (right). CM: compact myocardium, Epi: Epicardium. Scale bars: 20 μm. (C) Electron micrographs of cardiomyocytes in embryonic (left) and adult murine hearts (right). Asterisks point to sarcomeres, arrows to actin-anchoring adherens junctions, and arrowheads to desmin-anchoring desmosomes. TM: trabecular myocardium; CM: compact myocardium; m, mitochondrion. Scale bar: 1 μm.
3. Cardiogenesis
3.1. Contribution of Different Heart Fields
Lineage tracing and anatomical studies revealed that Mesp1+ cardiac progenitors appear in the anterior splanchnic mesoderm layer at the lateral sides of the primitive streak [34]. The cardiac progenitor cells are arranged in two different heart fields, i.e., the first heart field (FHF) and the second heart field (SHF). They develop in a temporally and spatially distinct manner and participate in the formation of discrete parts of the heart [35], each with a unique gene profile [36][37]. The FHF comprises cardiac progenitors, which appear first and differentiate rapidly. These fast-differentiating cells express the transcription factors NKX2-5, Tbx5, Hand1, and GATA4 as well as the chromatin regulatory factor BAF60c. Together, these factors drive the expression of sarcomeric proteins [38]. Morphologically, the cells in the lateral regions of the FHF migrate toward the midline and form a tubular heart at embryonic day (E) 8 in mice and during the third week of gestation in humans. Slowly differentiating but rapidly proliferating cardiac progenitors appear posterior to the FHF to form the SHF. They express the transcription factors Isl-1 and TBX-1 [39][40][41]. Following heart tube formation, the SHF cells migrate into the heart tube from the atrial and venous poles. The coordinated movement of SHF and FHF cells is facilitated via receptor-ligand interaction and leads to the elongation of the heart tube and the formation of the right ventricle and outflow tract [42]. The lumen of the heart is lined by endocardial cells, which are a unique type of endothelial cells, both in terms of their origin and differentiation capacities [43]. Endocardial cells appear at the same time as cardiac progenitors with whom they share a common origin [44]. The endocardial and myocardial cell layers are separated by an extracellular matrix, which is composed of hyaluronic acid, fibronectin, collagen IV, and proteoglycans [45][46][47][48]. Endocardial cells can undergo endothelial-to-mesenchymal transition and transform into mesenchymal endocardial cushion cells, which subsequently remodel to form cardiac valves and separate the outflow track into the pulmonary artery and aorta. Endocardial cells exhibit remarkable plasticity differentiating into various lineages including endothelial cells of the capillary network, adipocytes, fibroblasts, and hematopoietic cells [44][49]. At mid-gestation (E10.5), two types of cardiomyocytes are present in the ventricular myocardium. The majority (86%) are immature cardiomyocytes, which are primarily located in the compact myocardium [50]. They have a spherical shape and contain little cytoplasm with loosely arranged myofilaments. The remaining cardiomyocytes (14%) are elongated and have regularly arranged sarcomeres [50]. They are generated from the compact myocardium by proliferation and delamination of cells and make up the trabecular myocardium [51]. Adherens and desmosomal junctions are present in both cardiomyocyte types. But the spherical-shaped myocytes contain intercellular junctions along all sides whereas the trabecular myocytes restrict the junctions to intercalated discs for the most part (Figure 1B,C).3.2. Development of Epicardium and Epicardial-Derived Cells
Epicardial cells emerge from cell clusters that are referred to as the proepicardium (PE). The PE is located close to the liver primordium and sinus venosus. PE cells migrate toward the looped heart tube around E9.5, attach to the myocardium, and form the epicardial cell layer [52]. Prior to the attachment of epicardial cells to the myocardium at E9.5, the outer layer of cardiomyocytes is covered by a thin and patchy layer of fibronectin, laminin, and collagen IV, generating a basement membrane-like structure [53]. Epicardial cells initially contact myocytes directly through their α4 integrin receptor (CD49d), which binds VCAM-1 on adjacent cardiomyocytes [54]. Later, an extracellular matrix builds up between both cell layers. After formation of the epicardial layer, around E12 some epicardial cells undergo epithelial to mesenchymal transition (EMT), migrate into the sub-epicardial space and subsequently into the myocardium, where they can differentiate into fibroblasts, endothelial cells, and the smooth muscle cells surrounding arteries [55][56] as well as into mesenchymal stem cells [57]. At the same time, paracrine communication between epicardial and myocardial cells promotes myocardial growth [58]. In accordance, co-culture of embryonic epicardium-derived cells enhances the proliferation, maturation, and alignment of cardiomyocytes in vitro. This cross-talk involves increased expression of Cx43, N-cadherin, focal adhesion kinase, and sarcoplasmic reticulum Ca2+ ATPase [59]. Similarly, the promotion of structural and metabolic maturation of cardiomyocytes has been observed in co-cultures of cardiac fibroblasts (the derivatives of epicardial cells) with cardiomyocytes [60].3.3 Development of Intercellular Junctions in Embryonic Cardiomyocytes
N-cadherin is the main cadherin of classical adherens junctions that are formed in cardiac progenitors as they appear in the cardiac crescent [61][62]. Immature and spherical cardiomyocytes establish multiple contacts with neighboring cells through N-cadherin-based junctions and maintain them as they are required for cardiomyocyte differentiation and organization [30][63][64][65]. During cardiogenesis, the localization of N-cadherins to intercalated discs is closely followed by the appearance of desmosomes [66]. The formation of adherens junctions is a prerequisite for desmosome formation. Loss of N-cadherin, therefore, destabilizes intercalated discs and desmosomes in adult cardiomyocytes [67]. It is even more detrimental during cardiogenesis inducing the formation of a disorganized myocardium with adhesion-deficient cardiomyocytes, reduced trabeculation, loss of cell polarity, and outward migration of cardiomyocytes to the pericardial cavity [51][68]. After the establishment of adherens junctions, the spherical cardiomyocytes of the compact myocardium establish additional contact points by forming desmosomal adhesions with neighboring cardiomyocytes. Desmosomal proteins such as Pkp2 and Dsp can be identified in cardiomyocytes as early as embryonic day 9.5 [17][62]. This goes along with prominent shape changes from spheroidal to elongated. After the appearance of desmosomes, gap junctions are formed and expanded in the plasma membrane [62]. In mature cardiomyocytes of the adult, desmosomes are clustered together with other junctions in the intercalated disc region leaving the lateral membranes desmosome-free.References
- Paige, S.L.; Plonowska, K.; Xu, A.; Wu, S.M. Molecular regulation of cardiomyocyte differentiation. Circ. Res. 2015, 116, 341–353.
- Bartman, T.; Hove, J. Mechanics and function in heart morphogenesis. Dev. Dyn. 2005, 233, 373–381.
- Gaetani, R.; Zizzi, E.A.; Deriu, M.A.; Morbiducci, U.; Pesce, M.; Messina, E. When Stiffness Matters: Mechanosensing in Heart Development and Disease. Front. Cell Dev. Biol. 2020, 8, 334.
- Munch, J.; Abdelilah-Seyfried, S. Sensing and Responding of Cardiomyocytes to Changes of Tissue Stiffness in the Diseased Heart. Front. Cell Dev. Biol. 2021, 9, 642840.
- Xu, T.; Yang, Z.; Vatta, M.; Rampazzo, A.; Beffagna, G.; Pilichou, K.; Scherer, S.E.; Saffitz, J.; Kravitz, J.; Zareba, W.; et al. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 587–597.
- Gerull, B.; Brodehl, A. Genetic Animal Models for Arrhythmogenic Cardiomyopathy. Front. Physiol. 2020, 11, 624.
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802.
- Gao, S.; Puthenvedu, D.; Lombardi, R.; Chen, S.N. Established and Emerging Mechanisms in the Pathogenesis of Arrhythmogenic Cardiomyopathy: A Multifaceted Disease. Int. J. Mol. Sci. 2020, 21, 6320.
- Coscarella, I.L.; Landim-Vieira, M.; Pinto, J.R.; Chelko, S.P. Arrhythmogenic Cardiomyopathy: Exercise Pitfalls, Role of Connexin-43, and Moving beyond Antiarrhythmics. Int. J. Mol. Sci. 2022, 23, 8753.
- Thiene, G.; Basso, C.; Pilichou, K.; Bueno Marinas, M. Desmosomal Arrhythmogenic Cardiomyopathy: The Story Telling of a Genetically Determined Heart Muscle Disease. Biomedicines 2023, 11, 2018.
- Reisqs, J.B.; Moreau, A.; Sleiman, Y.; Boutjdir, M.; Richard, S.; Chevalier, P. Arrhythmogenic cardiomyopathy as a myogenic disease: Highlights from cardiomyocytes derived from human induced pluripotent stem cells. Front. Physiol. 2023, 14, 1191965.
- Peretto, G.; Sommariva, E.; Di Resta, C.; Rabino, M.; Villatore, A.; Lazzeroni, D.; Sala, S.; Pompilio, G.; Cooper, L.T. Myocardial Inflammation as a Manifestation of Genetic Cardiomyopathies: From Bedside to the Bench. Biomolecules 2023, 13, 646.
- Eshkind, L.; Tian, Q.; Schmidt, A.; Franke, W.W.; Windoffer, R.; Leube, R.E. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur. J. Cell Biol. 2002, 81, 592–598.
- Den, Z.; Cheng, X.; Merched-Sauvage, M.; Koch, P.J. Desmocollin 3 is required for pre-implantation development of the mouse embryo. J. Cell Sci. 2006, 119, 482–489.
- Park, J.; Son, Y.; Lee, N.G.; Lee, K.; Lee, D.G.; Song, J.; Lee, J.; Kim, S.; Cho, M.J.; Jang, J.H.; et al. DSG2 Is a Functional Cell Surface Marker for Identification and Isolation of Human Pluripotent Stem Cells. Stem. Cell Rep. 2018, 11, 115–127.
- Gallicano, G.I.; Bauer, C.; Fuchs, E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development 2001, 128, 929–941.
- Grossmann, K.S.; Grund, C.; Huelsken, J.; Behrend, M.; Erdmann, B.; Franke, W.W.; Birchmeier, W. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J. Cell Biol. 2004, 167, 149–160.
- Viragh, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; Kalman, F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat. Embryol. 1993, 188, 381–393.
- Eroglu, E.; Yen, C.Y.T.; Tsoi, Y.L.; Witman, N.; Elewa, A.; Joven Araus, A.; Wang, H.; Szattler, T.; Umeano, C.H.; Sohlmer, J.; et al. Epicardium-derived cells organize through tight junctions to replenish cardiac muscle in salamanders. Nat. Cell Biol. 2022, 24, 645–658.
- Matthes, S.A.; Taffet, S.; Delmar, M. Plakophilin-2 and the migration, differentiation and transformation of cells derived from the epicardium of neonatal rat hearts. Cell Commun. Adhes. 2011, 18, 73–84.
- Yuan, P.; Cheedipudi, S.M.; Rouhi, L.; Fan, S.; Simon, L.; Zhao, Z.; Hong, K.; Gurha, P.; Marian, A.J. Single-Cell RNA Sequencing Uncovers Paracrine Functions of the Epicardial-Derived Cells in Arrhythmogenic Cardiomyopathy. Circulation 2021, 143, 2169–2187.
- Kohela, A.; van Kampen, S.J.; Moens, T.; Wehrens, M.; Molenaar, B.; Boogerd, C.J.; Monshouwer-Kloots, J.; Perini, I.; Goumans, M.J.; Smits, A.M.; et al. Epicardial differentiation drives fibro-fatty remodeling in arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2021, 13, eabf2750.
- Reant, P.; Hauer, A.D.; Castelletti, S.; Pantazis, A.; Rosmini, S.; Cheang, M.H.; Peyrou, J.; Tome-Esteban, M.; Syrris, P.; Lafitte, S.; et al. Epicardial myocardial strain abnormalities may identify the earliest stages of arrhythmogenic cardiomyopathy. Int. J. Cardiovasc. Imaging 2016, 32, 593–601.
- Lombardi, R.; Chen, S.N.; Ruggiero, A.; Gurha, P.; Czernuszewicz, G.Z.; Willerson, J.T.; Marian, A.J. Cardiac Fibro-Adipocyte Progenitors Express Desmosome Proteins and Preferentially Differentiate to Adipocytes Upon Deletion of the Desmoplakin Gene. Circ. Res. 2016, 119, 41–54.
- Maione, A.S.; Faris, P.; Iengo, L.; Catto, V.; Bisonni, L.; Lodola, F.; Negri, S.; Casella, M.; Guarino, A.; Polvani, G.; et al. Ca(2+) dysregulation in cardiac stromal cells sustains fibro-adipose remodeling in Arrhythmogenic Cardiomyopathy and can be modulated by flecainide. J. Transl. Med. 2022, 20, 522.
- Gerull, B.; Brodehl, A. Insights Into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail. Rep. 2021, 18, 378–390.
- Quijada, P.; Trembley, M.A.; Small, E.M. The Role of the Epicardium During Heart Development and Repair. Circ. Res. 2020, 126, 377–394.
- Holthofer, B.; Windoffer, R.; Troyanovsky, S.; Leube, R.E. Structure and function of desmosomes. Int. Rev. Cytol. 2007, 264, 65–163.
- Hegazy, M.; Perl, A.L.; Svoboda, S.A.; Green, K.J. Desmosomal Cadherins in Health and Disease. Annu. Rev. Pathol. 2022, 17, 47–72.
- Hirschy, A.; Schatzmann, F.; Ehler, E.; Perriard, J.C. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev. Biol. 2006, 289, 430–441.
- Franke, W.W.; Borrmann, C.M.; Grund, C.; Pieperhoff, S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur. J. Cell Biol. 2006, 85, 69–82.
- Leo-Macias, A.; Liang, F.X.; Delmar, M. Ultrastructure of the intercellular space in adult murine ventricle revealed by quantitative tomographic electron microscopy. Cardiovasc. Res. 2015, 107, 442–452.
- Pieperhoff, S.; Franke, W.W. The area composita of adhering junctions connecting heart muscle cells of vertebrates - IV: Coalescence and amalgamation of desmosomal and adhaerens junction components - late processes in mammalian heart development. Eur. J. Cell Biol. 2007, 86, 377–391.
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835.
- Martin-Puig, S.; Wang, Z.; Chien, K.R. Lives of a heart cell: Tracing the origins of cardiac progenitors. Cell Stem Cell 2008, 2, 320–331.
- Lescroart, F.; Wang, X.; Lin, X.; Swedlund, B.; Gargouri, S.; Sanchez-Danes, A.; Moignard, V.; Dubois, C.; Paulissen, C.; Kinston, S.; et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 2018, 359, 1177–1181.
- Ma, Q.; Zhou, B.; Pu, W.T. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev. Biol. 2008, 323, 98–104.
- Lopez-Sanchez, C.; Garcia-Martinez, V. Molecular determinants of cardiac specification. Cardiovasc. Res. 2011, 91, 185–195.
- Bruneau, B.G.; Logan, M.; Davis, N.; Levi, T.; Tabin, C.J.; Seidman, J.G.; Seidman, C.E. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev. Biol. 1999, 211, 100–108.
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889.
- Galdos, F.X.; Wu, S.M. Single-Cell Delineation of Who's on First and Second Heart Fields During Development. Circ. Res. 2019, 125, 411–413.
- Xiong, H.; Luo, Y.; Yue, Y.; Zhang, J.; Ai, S.; Li, X.; Wang, X.; Zhang, Y.L.; Wei, Y.; Li, H.H.; et al. Single-Cell Transcriptomics Reveals Chemotaxis-Mediated Intraorgan Crosstalk During Cardiogenesis. Circ. Res. 2019, 125, 398–410.
- Misfeldt, A.M.; Boyle, S.C.; Tompkins, K.L.; Bautch, V.L.; Labosky, P.A.; Baldwin, H.S. Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors. Dev. Biol. 2009, 333, 78–89.
- Dye, B.; Lincoln, J. The Endocardium and Heart Valves. Cold Spring Harb. Perspect. Biol. 2020, 12, a036723.
- Silva, A.C.; Pereira, C.; Fonseca, A.; Pinto-do, O.P.; Nascimento, D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2020, 8, 621644.
- Rienks, M.; Papageorgiou, A.P.; Frangogiannis, N.G.; Heymans, S. Myocardial extracellular matrix: An ever-changing and diverse entity. Circ. Res. 2014, 114, 872–888.
- Jallerat, Q.; Feinberg, A.W. Extracellular Matrix Structure and Composition in the Early Four-Chambered Embryonic Heart. Cells 2020, 9, 285.
- Kalman, F.; Viragh, S.; Modis, L. Cell surface glycoconjugates and the extracellular matrix of the developing mouse embryo epicardium. Anat. Embryol. 1995, 191, 451–464.
- Zhang, H.; Lui, K.O.; Zhou, B. Endocardial Cell Plasticity in Cardiac Development, Diseases and Regeneration. Circ. Res. 2018, 122, 774–789.
- Zhang, F.; Pasumarthi, K.B. Ultrastructural and immunocharacterization of undifferentiated myocardial cells in the developing mouse heart. J. Cell Mol. Med. 2007, 11, 552–560.
- Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr. Cardiol. 2018, 39, 1082–1089.
- Männer, J.; Pérez-Pomares, J.M.; Macías, D.; Muñoz-Chápuli, R. The origin, formation and developmental significance of the epicardium: A review. Cells Tissues Organs 2001, 169, 89–103.
- Nahirney, P.C.; Mikawa, T.; Fischman, D.A. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev. Dyn. 2003, 227, 511–523.
- Yang, J.T.; Rayburn, H.; Hynes, R.O. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 1995, 121, 549–560.
- Cao, Y.; Duca, S.; Cao, J. Epicardium in Heart Development. Cold Spring Harb. Perspect. Biol. 2020, 12, a037192.
- Dettman, R.W.; Denetclaw, W., Jr.; Ordahl, C.P.; Bristow, J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 1998, 193, 169–181.
- Chong, J.J.; Chandrakanthan, V.; Xaymardan, M.; Asli, N.S.; Li, J.; Ahmed, I.; Heffernan, C.; Menon, M.K.; Scarlett, C.J.; Rashidianfar, A.; et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 2011, 9, 527–540.
- Boezio, G.L.M.; Zhao, S.; Gollin, J.; Priya, R.; Mansingh, S.; Guenther, S.; Fukuda, N.; Gunawan, F.; Stainier, D.Y.R. The developing epicardium regulates cardiac chamber morphogenesis by promoting cardiomyocyte growth. Dis. Model Mech. 2023, 16, dmm049571.
- Weeke-Klimp, A.; Bax, N.A.; Bellu, A.R.; Winter, E.M.; Vrolijk, J.; Plantinga, J.; Maas, S.; Brinker, M.; Mahtab, E.A.; Gittenberger-de Groot, A.C.; et al. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J. Mol. Cell Cardiol. 2010, 49, 606–616.
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; van Helden, R.W.J.; Krotenberg Garcia, A.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862–879 e811.
- Linask, K.K. N-cadherin localization in early heart development and polar expression of Na+,K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Dev. Biol. 1992, 151, 213–224.
- Navaratnam, V.; Kaufman, M.H.; Skepper, J.N.; Barton, S.; Guttridge, K.M. Differentiation of the myocardial rudiment of mouse embryos: An ultrastructural study including freeze-fracture replication. J. Anat. 1986, 146, 65–85.
- Linask, K.K.; Knudsen, K.A.; Gui, Y.H. N-cadherin-catenin interaction: Necessary component of cardiac cell compartmentalization during early vertebrate heart development. Dev. Biol. 1997, 185, 148–164.
- Imanaka-Yoshida, K.; Knudsen, K.A.; Linask, K.K. N-cadherin is required for the differentiation and initial myofibrillogenesis of chick cardiomyocytes. Cell Motil. Cytoskelet. 1998, 39, 52–62.
- Radice, G.L.; Rayburn, H.; Matsunami, H.; Knudsen, K.A.; Takeichi, M.; Hynes, R.O. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997, 181, 64–78.
- Vreeker, A.; van Stuijvenberg, L.; Hund, T.J.; Mohler, P.J.; Nikkels, P.G.; van Veen, T.A. Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS ONE 2014, 9, e94722.
- Kostetskii, I.; Li, J.; Xiong, Y.; Zhou, R.; Ferrari, V.A.; Patel, V.V.; Molkentin, J.D.; Radice, G.L. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 2005, 96, 346–354.
- Piven, O.O.; Kostetskii, I.E.; Macewicz, L.L.; Kolomiets, Y.M.; Radice, G.L.; Lukash, L.L. Requirement for N-cadherin-catenin complex in heart development. Exp. Biol. Med. 2011, 236, 816–822.
More
