Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Seong-Gon Kim and Version 2 by Catherine Yang.
The potential of sericin, a protein derived from silkworms, is explored in bone graft applications. Sericin’s biocompatibility, hydrophilic nature, and cost-effectiveness make it a promising candidate for enhancing traditional graft materials. Its antioxidant, anti-inflammatory, and UV-resistant properties contribute to a healthier bone-healing environment, and its incorporation into 3D-printed grafts could lead to personalized medical solutions.
- sericin
- bone graft applications
- biocompatibility
- immune responses
1. Introduction
Bone grafting is a surgical procedure that is designed to fix problems associated with bones or joints. This operation plays an indispensable role in various medical fields, ranging from orthopedics, dental procedures, and plastic surgery to treatments of trauma, disease, or skeletal abnormalities. The procedure traditionally involves the transplantation of bone tissue, known as a graft, to repair and rebuild diseased or damaged bones [1]. A bone graft can fill a void where bone is absent, providing a scaffold upon which new bone can grow [2]. This not only stimulates bone growth but also aids in maintaining bone strength and structure [3]. Therefore, bone grafts hold immense importance in healing complex fractures, aiding bone growth around surgically implanted devices, and promoting fusion between bony fragments [4].
Traditionally, the sources of bone graft materials have been broadly classified into four categories: autografts (from the patient’s own body), allografts (from a human donor), xenografts (from a nonhuman species), and synthetic substitutes [5]. Autografts, derived from the patient’s own body, are considered the “gold standard” for bone grafts due to their high biocompatibility and osteoinductive properties [6]. However, the harvesting procedure may lead to donor site morbidity, and there are limitations on the available volume. Allografts and xenografts, obtained from human donors and animal species, respectively, bypass the issue of limited material availability [7][8][9]. However, they carry risks of immunogenicity, disease transmission, and may lack the osteoinductive properties found in autografts. Synthetic substitutes, on the other hand, such as hydroxyapatite, bioactive glasses, and polymers, can be manufactured in large quantities and tailored to specific needs [10][11]. Nonetheless, these substitutes may not fully replicate the biological properties of natural bone, potentially affecting graft success. Thus, while each of these traditional materials has played a significant role in bone grafting, they also come with their own set of limitations. These issues highlight the need for exploring new materials for bone grafts, ones that can combine the benefits of the traditional materials while overcoming their shortcomings [12].
Sericin is a natural protein primarily derived from the cocoon of the Bombyx mori silkworm, an insect that has been at the heart of silk production for thousands of years [13]. It is secreted by the silkworm during the spinning of its cocoon and is used as a “bonding” agent, accounting for approximately 20–30% of the total weight of the silk cocoon [14]. Additionally, spiders also produce sericin [15].
What makes sericin unique is its “glue-like” characteristic, which allows it to bind the two fibroin filaments together, forming a single silk thread [16]. This feature comes from its molecular structure, which is characterized by its high content of polar amino acids, including serine, aspartic acid, and threonine [17][18]. These polar amino acids form hydrogen bonds with the surrounding water molecules, leading to its adhesive and gel-forming properties [19].
In addition to its bonding capabilities, sericin has gained attention in the biomedical field for its exceptional biocompatibility and biodegradability [17][20]. Biocompatibility refers to the ability of a material to perform its desired function, without eliciting any undesirable local or systemic effects in the host, while biodegradability ensures that the material can be broken down into harmless products by biological systems [20]. These characteristics make sericin a promising material for various biomedical applications, including tissue engineering and drug delivery (Figure 1).
Figure 1. The biomedical field is increasingly recognizing sericin for its outstanding biocompatibility and biodegradability. These attributes position sericin as a potential candidate for diverse biomedical uses, such as tissue engineering and drug delivery.
Moreover, sericin is also known for its antioxidant, UV-resistant, and antibacterial properties [17]. The antioxidant property of sericin helps in neutralizing harmful free radicals in the body, thereby preventing cell damage [21]. Its UV-resistant property provides protection against ultraviolet rays, making it useful in the cosmetics industry [22]. Additionally, its antibacterial property inhibits the growth of certain bacteria, providing potential applications in wound healing [23]. Given these unique properties, sericin’s application in the field of bone grafting presents an intriguing direction for future research.
2. Sericin Properties Relevant to Bone Grafting
The biocompatibility of sericin, which is derived from the B. mori silkworm cocoon, has been extensively investigated in several studies [15][16][17]. As a protein, sericin possesses inherent affinity with bodily tissues, thereby reducing the likelihood of any detrimental immune responses upon its introduction into the body [24]. This characteristic renders sericin an extremely promising candidate for various biomedical applications, encompassing drug delivery, tissue engineering, and wound healing [25].
The unique properties of sericin have been the subject of scientific exploration due to its potential in biomedical research. It has been found that sericin displays a remarkable ability to integrate with bodily tissues, owing to its natural composition and structure [17]. This integration not only minimizes any potential adverse immune reactions but also ensures a more seamless and efficient interaction between the material and the host organism [24].
The biocompatibility of sericin is of particular interest in the field of drug delivery. Sericin-based carriers have been developed to encapsulate and transport various therapeutic agents, such as drugs and biologics, to target sites within the body [25]. The compatibility of sericin with bodily tissues promotes the controlled release of these agents, enhancing their therapeutic efficacy while reducing the risk of adverse effects [17]. Furthermore, sericin’s ability to interact with cells at the molecular level allows for targeted drug delivery, enabling precise localization of therapeutic agents to specific tissues or cells [24].
Sericin’s potential for tissue engineering applications is also noteworthy. Its biocompatibility and affinity with bodily tissues make sericin an ideal material for constructing scaffolds that support cell growth and tissue regeneration [25]. By providing a conducive environment for cell attachment, proliferation, and differentiation, sericin-based scaffolds can facilitate the regeneration of damaged or diseased tissues [17]. Additionally, sericin’s ability to modulate cellular behavior and promote angiogenesis further enhances its suitability for tissue engineering applications [24].
Wound healing is another area where sericin’s properties can be harnessed. The biocompatibility and affinity of sericin with bodily tissues promote wound healing by facilitating the formation of a favorable microenvironment for tissue regeneration [25]. Sericin-based dressings have been shown to accelerate wound closure, reduce scar formation, and enhance tissue regeneration [21]. Furthermore, sericin’s antimicrobial properties contribute to the prevention of infection, a common complication in wound healing [23].
In the field of bone grafting, the biocompatibility of the graft material is of utmost importance. It is crucial for the material to not only interact safely with the bone tissues but also facilitate the growth and development of new bone cells. Research has indicated that sericin, owing to its proteinaceous nature and high biocompatibility, shows promise in promoting cellular adhesion and proliferation [24][26]. This makes it an excellent candidate for bone graft applications. Additionally, sericin is biodegradable [20], which means that it can be gradually broken down and replaced by the patient’s own cells. This feature further reduces the risk of long-term foreign body reactions.
This sets sericin apart from many synthetic materials commonly used in bone grafting, as these materials may persist in the body and lead to chronic inflammation or other complications. By contrast, sericin’s biodegradability ensures a more seamless integration with the surrounding tissues, minimizing potential adverse effects. Further research is needed to fully explore the potential of sericin in bone grafting applications. However, the preliminary findings regarding its biocompatibility and ability to promote cellular adhesion and proliferation are promising [24][26]. If these initial results are confirmed and expanded upon, sericin has the potential to revolutionize the field of bone grafting by providing a safer and more effective alternative to synthetic materials.
Sericin, a protein derived from the Antheraea pernyi silkworm, has been found to have a significant impact on the formation of hydroxyapatite (HAp) nanoneedle-like crystals [27]. This process is influenced by the concentration of sericin as well as the length of time allowed for mineralization [28]. In vitro assays have demonstrated that mineralized sericin/HAp composites exhibit a higher level of osteogenic differentiation in bone marrow stem cells (BMSCs) compared to nonmineralized composites [28]. One of the key mechanisms by which sericin promotes this mineralization process is through its stimulation of collagen production. Collagen is a crucial protein in the composition of the bone matrix [29]. Sericin’s ability to enhance collagen production is thought to play a significant role in facilitating the formation and growth of HAp crystals. Furthermore, the amino acids present in sericin, particularly glutamic acid and aspartic acid, have been identified as key players in regulating the formation and growth of HAp crystals [30][31]. These amino acids have the unique ability to attract calcium and phosphate ions, thereby increasing the local supersaturation and creating an environment conducive to the nucleation and growth of HAp crystals. The findings regarding sericin’s ability to promote the formation of HAp crystals and enhance osteogenic differentiation in BMSCs hold great promise for the field of bone grafting. The potential of sericin as a biomaterial in bone graft applications should be further explored through rigorous research and experimentation. If confirmed, sericin could revolutionize the field by providing a biocompatible and effective alternative to existing synthetic materials.
The use of sericin-based biomaterials, such as sericin-coated titanium and sericin nanofiber, has shown promising results in promoting osseointegration [32] and osteogenic differentiation [28]. In a study, it was observed that sericin extract from B. mori, when applied at a concentration of 40 μg/mL, significantly increased the proliferation of osteoblast cells by up to 135% compared to the untreated control [33]. Furthermore, it was found that the unmodified form of sericin possesses dual-functional properties, displaying both cytocompatibility towards osteoblast cells and antibacterial/biofilm activity against Staphylococcus aureus [33]. These significant findings have paved the way for the development of biomaterials based on sericin for bone tissue engineering applications.
3. Studies on Sericin in Bone Grafting
Silk mat, derived from the silkworm cocoon of B. mori, contains various proteins, including sericin [34]. It has been observed that when silk mat is used as a membrane for guided bone regeneration, it exhibits comparable new bone formation to that of collagen membrane [35]. When silk mat is placed in phosphate-buffered saline (PBS), a soluble fraction is released into the saline (Figure 2). This solution, after protein concentration determination, is applied to macrophages to examine differentially expressed genes using cDNA microarray analysis [36]. Among the genes studied, bone morphogenetic protein-2 (BMP-2) has shown increased expression upon administration of the silk mat-derived solution [36]. BMP-2 is a potent osteogenic protein [37]. It is hypothesized that a protein present in this solution may stimulate macrophages to secrete BMP-2. Isolation of this protein could potentially be advantageous for bone regeneration purposes.
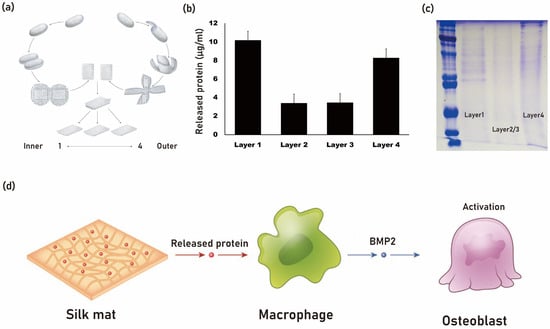
Figure 2. The silk mat, derived from silkworm cocoon (a), exhibits a varied protein composition from its inner to outer layers. The innermost (layer 1) and outermost layers (layer 4) release a higher amount of protein (b). When the silk mat is immersed in phosphate-buffered saline (PBS), a soluble protein fraction is released, as depicted in the SDS page gel (c). It is theorized that a protein present in the solution may induce macrophages to release BMP-2, which can potentially enhance bone regeneration by stimulating osteoblasts (d).
Through the application of different degumming temperatures, it is possible to obtain differential protein profiles from the silk cocoon [38]. By utilizing a molecular weight-based filter, these proteins can be classified into detailed groups [38]. As a result of this process, the BMP-2-inducing protein present in the silk mat was identified as sericin [38]. Sericin, being an insect-derived protein, is considered a foreign protein for mammals [39]. Foreign proteins often elicit immune responses through the recognition of pattern-recognizing receptors, such as Toll-like receptors (TLRs), by immune cells (Figure 3). In the case of sericin, when it is applied to macrophages, it is recognized by the TLRs present on these immune cells [38][39].
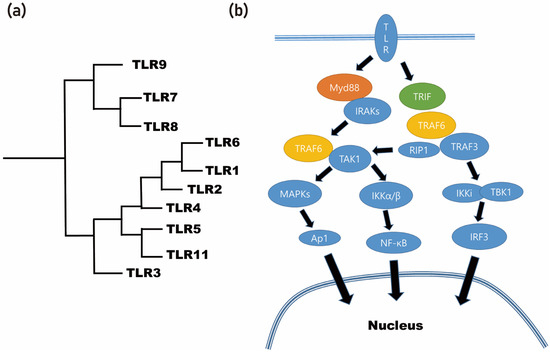
Figure 3. Foreign proteins often elicit immune responses through the recognition of Toll-like receptors (TLRs) by immune cells. (a) Phylogenetic analysis of TLR. (b) Summarized signaling pathway of Toll-like receptor (MyD88: myeloid differentiation primary response 88; TRIF: toll/interleukin-1 receptor (TIR) domain-containing adapter-inducing interferon-β; IRAKs: interleukin-1 receptor-associated kinases; TRAF6: tumor necrosis factor (TNF) receptor-associated factor; TAK1: TRAF6-induced transforming growth factor beta-activated kinase 1; MAPKs: mitogen-activated protein kinases; NF-κB: nuclear factor-κB; ERK1/2: extracellular signal-regulated kinase 1/2; JNK: c-Jun N-terminal kinase; AP-1: activator protein-1; RIP1: receptor-interacting serine/threonine protein kinase 1; IKKi: IκB kinase-I; TBK1: TRAF family member-associated NF-κB activator (TANK)-binding kinase 1; IRF3: interferon regulatory transcription factor 3).
Consequently, the BMP-2-inducing effect of sericin is mediated by the TLRs expressed on macrophages [39]. It is important to note that TLRs are pattern-recognizing receptors, and therefore, the conformation of sericin protein could potentially influence the expression level of BMP-2 [38][40]. This finding regarding the role of sericin in inducing BMP-2 expression sheds light on the potential mechanisms underlying the bone regenerative properties of silk mat [36]. Understanding the interaction between sericin and TLRs provides valuable insights for the development of novel approaches for bone regeneration purposes.
The degumming process plays a crucial role in influencing the conformation of sericin, a protein derived from silk (Figure 4). It has been observed that using a degumming temperature below 65 ºC results in a greater abundance of β-sheet conformation in sericin compared to using temperatures above 100 °C [38][41]. Drying sericin may extend its storage time, and β-sheet crystallization also increases with decreasing temperature [41]. It has been found that sericin with a rich β-sheet conformation exhibits a higher ability to induce the expression of BMP-2 in macrophages, compared to sericin with other conformations [40].
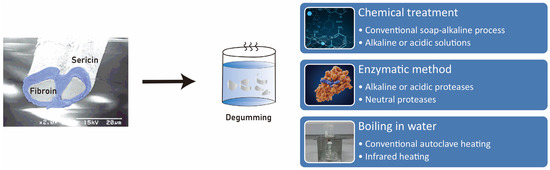
Figure 4. The “degumming process” refers to the removal of sericin from the silkworm cocoon. There are various types of degumming processes. This process is critical as it significantly influences the conformation of sericin.
However, it is important to note that the low-temperature method of degumming has been found to have a lower yielding ratio compared to the high-temperature method [40]. This limitation can be overcome by employing alternative degumming solutions to produce sericin with a high content of β-sheet conformation. For example, studies have demonstrated that using a solution of 70% ethanol [18], formic acid [18], or 2% 4-hexylresorcinol (4HR) [40] can stabilize the protein at high temperatures and yield a greater amount of β-sheet rich sericin, in comparison to using distilled water. These alternative degumming solutions have shown promising results in promoting the formation of β-sheet structures in sericin. Ethanol, formic acid, and 4HR have been found to effectively stabilize the protein during the degumming process, leading to a higher content of β-sheet conformation. This is crucial, as the β-sheet structure is associated with the superior mechanical properties and stability of sericin-based materials.
The use of ethanol as a degumming solution has been reported to enhance the formation of β-sheet structures in sericin [18]. Ethanol acts as a denaturant, disrupting the non-covalent interactions and hydrogen bonds in the protein, promoting the rearrangement of the protein chains into β-sheet structures. This results in a higher content of β-sheet conformation in the extracted sericin. Similarly, formic acid has been shown to have a stabilizing effect on sericin, promoting the formation of β-sheet structures [18]. Formic acid acts as a chemical denaturant, altering the secondary structure of the protein and facilitating the rearrangement of the protein chains into β-sheet conformations. This leads to an increased content of β-sheet structures in the sericin extract. Another alternative degumming solution, 4HR, has been found to effectively stabilize sericin at high temperatures and promote the formation of β-sheet structures [40]. 4HR acts as a chemical chaperone, interacting with the protein molecules and facilitating the rearrangement of the protein chains into β-sheet conformations [42]. This results in a higher yield of β-sheet rich sericin.
Immunomodulation is a tissue engineering technique that involves the use of molecules to manipulate the immune response in a desired direction [43]. In the context of bone tissue regeneration, bone resident macrophages play a crucial role [44]. It has been observed that sericin, a protein derived from silk, can induce the expression of BMP-2 in macrophages [38]. This is important because BMP-2, when derived from macrophages, can stimulate resting osteoblasts, and activate them (Figure 5). In coculture experiments, the administration of sericin to macrophages was shown to activate osteoblasts [38]. Furthermore, when a sericin-incorporated gelatin sponge is implanted into a bony defect, there is a higher level of new bone formation compared to when using a gelatin sponge alone [38][40]. Additionally, the expression level of BMP-2 is higher in the tissue of the sericin-incorporated gelatin sponge group compared to the other groups [38].
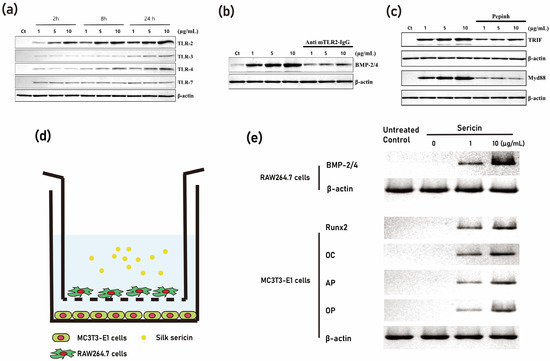
Figure 5. Activation of the TLR-mediated pathway through the application of sericin. (a) demonstrates increased expression levels of TLR-2, TLR-3, and TLR-4 induced by sericin. (b) shows that the application of mouse TLR-2 neutralizing antibodies results in a decrease in the level of BMP-2/4 expression initiated by sericin. (c) reflects that the application of an inhibitory peptide (Pepinh) for TRIF and MyD88 yields a similar effect to that of TLR-2 antibodies. A coculture experiment with RAW264.7 cells (macrophage) and MC3T3-E1 cells (osteoblast) supports these findings. (d) provides a schematic representation of the coculture system. (e) reveals that sericin application boosts BMP-2/4 levels in RAW264.7 cells. In MC3T3-E1 cells, the expression levels of Runx2, osteocalcin, and osteonectin were found to be higher in the RAW264.7 cells of the sericin application group compared to the RAW264.7 cells not treated with sericin (modified from author’s own publication [38]).
B. mori, commonly known as the silkworm, encompasses various subspecies, and these subspecies may exhibit differences in the amino acid sequence of sericin, a protein derived from silk. In a comparative analysis of three different subspecies, namely Baegokjam, Yeonnokjam, and Goldensilk, it was found that the expression level of BMP-2, an important factor in bone regeneration, was highest in the Baegokjam subspecies [45]. Sericinjam is a genetically modified silkworm that has been selectively bred to produce sericin exclusively [46]. This selective breeding allows sericin to be obtained without the need for the traditional degumming process [47]. However, it should be noted that natural sericin obtained from Sericinjam cannot be directly grafted into the body without undergoing a cleaning process. Therefore, for the application of sericin from Sericinjam as a bone graft material, special processing techniques are required to ensure its effectiveness.
References
- Park, H.I.; Lee, J.H.; Lee, S.J. The comprehensive on-demand 3D bio-printing for composite reconstruction of mandibular defects. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 31.
- Kim, S.G. Multiple ways for the same destination: Bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 9.
- Nelke, K.; Łuczak, K.; Janeczek, M.; Pasicka, E.; Żak, K.; Łukaszewski, M.; Jadach, R.; Dobrzyński, M. Fresh–frozen allogenic bone graft usage in treatment of an odontogenic keratocyst in the mandible. Appl. Sci. 2023, 13, 1234.
- Salah, M.; Naini, F.B. Exosomes in craniofacial tissue reconstruction. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 27.
- Kim, S.; Hwang, J.; Cho, B.H.; Kim, Y.; Lee, J.Y. Three-dimensional analysis of bone volume change at donor sites in mandibular body bone block grafts by a computer-assisted automatic registration method: A retrospective study. Appl. Sci. 2022, 12, 7261.
- Kim, M.G.; Lee, J.H.; Kim, G.C.; Hwang, D.S.; Kim, C.H.; Kim, B.J.; Kim, J.H.; Kim, U.K. The effect of autogenous tooth bone graft material without organic matter and type I collagen treatment on bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 17.
- Choi, W.H.; Kim, Y.D.; Song, J.M.; Shin, S.H. Comparative study of bone regeneration using fibrin sealant with xenograft in rabbit sinus: Pilot study. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 5.
- Pfaffeneder-Mantai, F.; Meller, O.; Schneider, B.; Bloch, J.; Bytyqi, D.; Sutter, W.; Turhani, D. Specially designed and CAD/CAM manufactured allogeneic bone blocks using for augmentation of a highly atrophic maxilla show a stable base for an all-on-six treatment concept: A case report. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 21.
- Reis, C.H.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.; et al. Effects of a biocomplex formed by two scaffold biomaterials, hydroxyapatite/tricalcium phosphate ceramic and fibrin biopolymer, with photobiomodulation, on bone repair. Polymers 2022, 14, 2075.
- Vani, T.M.; Paramashivaiah, R.; Prabhuji, M.L.; Peeran, S.W.; Fageeh, H.; Tasleem, R.; Bahamdan, G.K.; Aldosari, L.I.; Bhavikatti, S.K.; Scardina, G.A. Regeneration of intrabony defects with nano hydroxyapatite graft, derived from eggshell along with periosteum as barrier membrane under magnification—An interventional study. Appl. Sci. 2023, 13, 1693.
- Negut, I.; Ristoscu, C. Bioactive glasses for soft and hard tissue healing applications—A short review. Appl. Sci. 2023, 13, 6151.
- Di-Stefano, D.A.; Orlando, F.; Ottobelli, M.; Fiori, D.; Garagiola, U. A comparison between anorganic bone and collagen-preserving bone xenografts for alveolar ridge preservation: Systematic review and future perspectives. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 24.
- Ji, Y.; Zhang, X.; Chen, Z.; Xiao, Y.; Li, S.; Gu, J.; Hu, H.; Cheng, G. Silk sericin enrichment through electrodeposition and carbonous materials for the removal of methylene blue from aqueous solution. Int. J. Mol. Sci. 2022, 23, 1668.
- Wang, W.H.; Lin, W.S.; Shih, C.H.; Chen, C.Y.; Kuo, S.H.; Li, W.L.; Lin, Y.S. Functionality of silk cocoon (Bombyx mori L.) sericin extracts obtained through high-temperature hydrothermal method. Materials 2021, 14, 5314.
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416.
- Seo, S.J.; Das, G.; Shin, H.S.; Patra, J.K. Silk sericin protein materials: Characteristics and applications in food-sector industries. Int. J. Mol. Sci. 2023, 24, 4951.
- Kunz, R.I.; Brancalhão, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 19.
- Jo, Y.N.; Um, I.C. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int. J. Biol. Macromol. 2015, 78, 287–295.
- Guo, K.; Zhang, X.; Zhao, D.; Qin, L.; Jiang, W.; Hu, W.; Liu, X.; Xia, Q.; Dong, Z.; Zhao, P. Identification and characterization of sericin5 reveals non-cocoon silk sericin components with high β-sheet content and adhesive strength. Acta Biomater. 2022, 150, 96–110.
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524.
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk sericin: A promising sustainable biomaterial for biomedical and pharmaceutical applications. Polymers 2022, 14, 4931.
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012, 347, 783–794.
- Mehdi, M.; Qiu, H.; Dai, B.; Qureshi, R.F.; Hussain, S.; Yousif, M.; Gao, P.; Khatri, Z. Green synthesis and incorporation of sericin silver nanoclusters into electrospun ultrafine cellulose acetate fibers for anti-bacterial applications. Polymers 2021, 13, 1411.
- Cherng, J.H.; Chang, S.J.; Chiu, Y.K.; Chiu, Y.H.; Fang, T.J.; Chen, H.C. Low molecular weight sericin enhances the in vitro of immunological modulation and cell migration. Front. Bioeng. Biotechnol. 2022, 10, 925197.
- Jo, Y.Y.; Kweon, H.; Oh, J.H. Sericin for tissue engineering. Appl. Sci. 2020, 10, 8457.
- Lee, J.H.; Bae, Y.S.; Kim, S.J.; Song, D.W.; Park, Y.H.; Bae, D.G.; Choi, J.H.; Um, I.C. Preparation of new natural silk non-woven fabrics by using adhesion characteristics of sericin and their characterization. Int. J. Biol. Macromol. 2018, 106, 39–47.
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent advances in silk sericin/calcium phosphate biomaterials. Front. Mater. 2020, 7, 24.
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYand, H. Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules 2014, 15, 1185–1193.
- Aramwit, P. Bio-response to silk sericin. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2014; pp. 299–329.
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693.
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462.
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864.
- Noosak, C.; Jantorn, P.; Meesane, J.; Voravuthikunchai, S.; Saeloh, D. Dual-functional bioactive silk sericin for osteoblast responses and osteomyelitis treatment. PLoS ONE 2022, 17, e0264795.
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Baek, K.; Kim, M.K.; Kim, S.G.; Chae, W.S.; Choi, J.Y.; Rotaru, H. Bone regeneration is associated with the concentration of tumour necrosis factor-α induced by sericin released from a silk mat. Sci. Rep. 2017, 7, 15589.
- Ha, Y.Y.; Park, Y.W.; Kweon, H.; Jo, Y.Y.; Kim, S.G. Comparison of the physical properties and in vivo bioactivities of silkworm-cocoon-derived silk membrane, collagen membrane, and polytetrafluoroethylene membrane for guided bone regeneration. Macromol. Res. 2014, 22, 1018–1023.
- Kim, J.W.; Jo, Y.Y.; Kweon, H.Y.; Kim, D.W.; Kim, S.G. The effects of proteins released from silk mat layers on macrophages. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 10.
- Han, J.J.; Chang, A.R.; Ahn, J.; Jung, S.; Hong, J.; Oh, H.K.; Hwang, S.J. Efficacy and safety of rhBMP/β-TCP in alveolar ridge preservation: A multicenter, randomized, open-label, comparative, investigator-blinded clinical trial. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 42.
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Baek, K.; Chae, W.S.; Kang, Y.J.; Oh, J.H.; Kim, S.G.; Garagiola, U. Silk sericin application increases bone morphogenic protein-2/4 expression via a toll-like receptor-mediated pathway. Int. J. Biol. Macromol. 2021, 190, 607–617.
- Kim, S.G.; Kweon, H.; Jo, Y.Y. Toll-like receptor and silk sericin for tissue engineering. Int. J. Indust. Entomol. 2021, 42, 1–6.
- Lee, J.H.; Kweon, H.; Oh, J.H.; Kang, Y.J.; Kim, D.W.; Yang, W.G.; Chae, W.S.; Kim, S.G. 4-Hexylresorcinol treatment before degumming increases the β-sheet structure of silk sericin and BMP-2 expression in RAW264.7 cells. Int. J. Mol. Sci. 2022, 24, 150.
- Jo, Y.N.; Park, B.D.; Um, I.C. Effect of storage and drying temperature on the gelation behavior and structural characteristics of sericin. Int. J. Biol. Macromol. 2015, 81, 936–941.
- Kang, Y.J.; Yang, W.G.; Chae, W.S.; Kim, D.W.; Kim, S.G.; Rotaru, H. Administration of 4-hexylresorcinol increases p53-mediated transcriptional activity in oral cancer cells with the p53 mutation. Oncol. Rep. 2022, 48, 160.
- Kim, S.G. Immunomodulation for maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 5.
- Weivoda, M.M.; Bradley, E.W. Macrophages and bone remodeling. J. Bone Miner. Res. 2023, 38, 359–369.
- Lee, J.H.; Oh, J.H.; Kim, D.W.; Kim, S.G.; Kweon, H. The difference in bone morphogenic protein-2 expression level among Bombyx mori subspecies. Int. J. Indust. Entomol. 2022, 45, 78–83.
- Kweon, H.; Yeo, J.H.; Kim, K.Y.; Kim, Y.S.; Song, H.S.; Kim, S.J.; Woo, S.; Han, S.; Lee, K.G. Characteristics of silk sericin extracted from Sericinjam. Int. J. Indust. Entomol. 2009, 18, 121–124.
- Choi, Y.Y.; Kim, S.W.; Kim, K.Y.; Um, I.C. Dissolution, crystallilnity, and mechanical properties of silk sericin from Sericinjam silkworm cocoons. Int. J. Indust. Entomol. 2023, 46, 9–15.
More
