Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Charalampos Konstantinou.
Microbially induced carbonate precipitation (MICP) refers to a natural biochemical phenomenon wherein micro-organisms stimulate the formation of calcium carbonate precipitation.
- MICP
- biocementation
- hydraulic conductivity
- porosity
1. Introduction
Microbially induced carbonate precipitation (MICP) refers to a natural biochemical phenomenon wherein micro-organisms stimulate the formation of calcium carbonate precipitation. Microbes trigger the formation of carbonate precipitation through various metabolic pathways, including photosynthesis, ureolysis, ammonification, denitrification, sulfate reduction, anaerobic sulfide oxidation, and methane oxidation. These pathways involve mechanisms that raise pH levels and increase the presence of dissolved inorganic carbon (DIC) [1]. Biocementation techniques having MICP as the underlying biochemical mechanism responsible for calcium carbonate generation are widely employed to achieve various outcomes with the main aim usually being the solidification of sand. The precipitating calcium carbonate forms the bonding material between particles. The key metabolic pathway is the one of urea hydrolysis, which is used in most relevant studies. The process involves three distinct stages: (i) introduction of bacteria into the medium, followed by (ii) the injection of a cementation solution containing urea and a calcium source, which (iii) results in the precipitation of the cementing agent that binds the sand particles, leading to an increase in both strength and stiffness [2,3][2][3].
It is described by two chemical equations, the first of which (see chemical Equation (1)) involves the hydrolysis of urea, which is a slow and irreversible reaction influenced by pH and various environmental conditions. The reaction results in the release of ammonium ions (NH4+) and carbonate ions (CO32−) in the form of inorganic carbon. The presence of ammonium ions leads to a rise of the pH of the solution due to their basic nature. The elevated pH creates an environment conducive to calcium carbonate precipitation. The carbonate ions, on the other hand, are essential for MICP. Urea hydrolysis, therefore, is considered one of the most effective reactions for MICP since it increases the alkalinity and concentration of dissolved inorganic carbon in the solution, as explained before. Then, as a result of the pH elevation, the carbonate ions react with the available calcium ions in the environment (see chemical Equation (2)), leading to precipitation at particle contacts. The reaction is enhanced within the alkaline environment and in the presence of inorganic carbon, and it occurs when the solution becomes supersaturated. Supersaturation can be achieved by introducing ionic salts that act as reactant sources, such as Na2CO3 and CaCl2 [4].
CO(NH
CO(NH
2
)
2
→ 2NH
4+
+ CO
32
CaCl
(1)
CaCl
2
+ CO
32−
→ CaCO
3
+ 2Cl
−
(2)
Figure 1 serves as a comprehensive visual representation that intricately illustrates the step-by-step progression of the MICP process. Beginning with the introduction of bacteria into the medium, enzymatic action takes place whereby urea is hydrolyzed by the micro-organisms. This enzymatic activity yields ammonium ions (which, in turn, elevates pH) and carbonate ions as products. Positively charged calcium ions are attracted to the negatively charged bacterial walls, leading to the precipitation of calcium carbonate (CaCO3) in solid form due to the alkaline environment and supersaturation around the cells. This precipitate formation is facilitated by the alkaline environment brought about by the elevated pH and the saturation of calcium and carbonate ions. Once the desired amount of calcite is achieved, the nutrition is halted, causing bacteria to die. As depicted in the MicroCT image in Figure 1, rock-like materials are generated which preserve to a great extent their pore network, and the calcium carbonate is found within this pore network or on and around the grains.
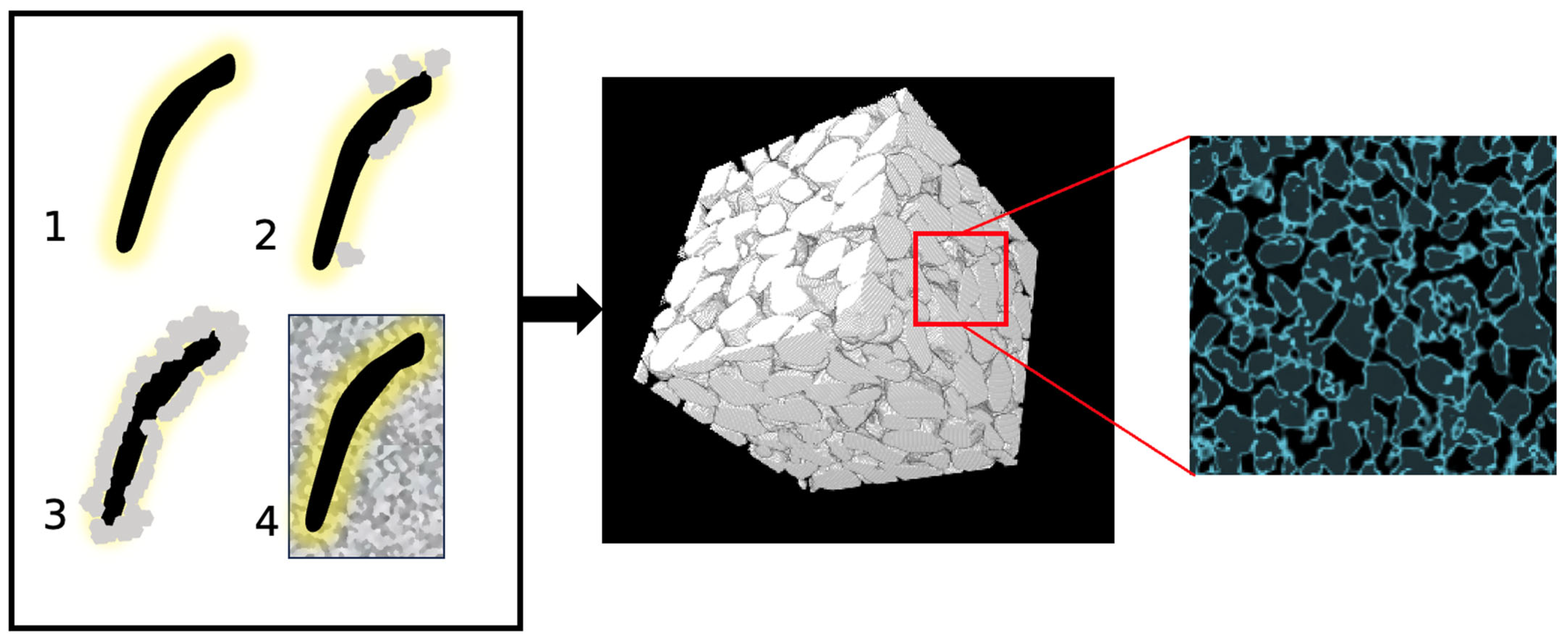
Figure 1. The biologically induced calcite process in sand [5]. 1. Bacteria hydrolyse urea to raise the pH of the system. 2–3. Cations are attracted to bacteria due to their negative-charged wall. The alkaline environment formed and the supersaturation around the cells results in the calcium carbonate precipitation into solid form. 4. Cement nucleates around bacterial cells within the pore space of the granular network. An example of a MicroCT image is shown with cementation highlighted in blue.
The carbonate precipitation involves several processes, including nucleation, transformation, and crystal growth. The reaction can produce different forms of calcium carbonate, including transformable (calcium carbonate forms that can undergo changes in their crystalline structure over time), unstable (forms that are not thermodynamically favored and can, eventually, transform into more stable structures), and stable forms (which represent the thermodynamically favoured, enduring configurations of calcium carbonate), depending on the specific conditions of the reaction (e.g., rhomboidal or amorphous calcite, vaterite, etc.). The specific form of calcium carbonate produced through MICP can impact the physical properties of the solidified material. For instance, some forms might have greater hardness or compressive strength than others. Also, stable forms of calcium carbonate are less prone to further transformations over time, making them more enduring and resistant to changes in environmental conditions while unstable forms are likely to undergo phase transformations, which could affect the stability and longevity of the solidified material.
According to Rahman et al. [6], the method finds its routes in the work by Gollapudi et al. [7] back in 1995 aiming at controlling leaching of groundwater contaminants. Then, in 1999, Stocks-Fisher et al. [8] assessed the properties of the carbonate crystals resulting from MICP. In 2004, Whiffin [9] proposed the method for soil improvement and, then, the method received great interest in the field of geotechnical engineering involving even the upscaling of the process [10]. Initially employed for purposes like stabilizing soil against sliding, preventing liquefaction (prohibiting the onset of fluidized behaviour of cohesionless soil during dynamic loading), and erosion (prevention of movement, and transportation of soil particles by various external forces), microbially induced carbonate precipitation (MICP) has been extensively studied and applied in various fields imitating sedimentation processes and resembling natural geological formations with pore-filling carbonate cements [11,12][11][12]. Its applications include strengthening of soils to improve their mechanical properties with applications in tunneling to avoid wall collapsing and cave-ins during excavation, foundation engineering, self-healing of cracks in concrete and rocks restoring the structural integrity, and to reinforce methane hydrate layers beneath the deep-ocean floor during the depressurization process when producing methane gas, reducing the risk of destabilization during gas extraction [13,14,15,16,17,18,19][13][14][15][16][17][18][19]. In recent times, MICP has also found new applications in areas related to ocean and marine engineering like providing erosion resistance against wave actions, preventing corrosion in marine environments, and contributing to the construction of ocean islands and reefs, showcasing its adaptability and potential in addressing a range of challenges unique to aquatic environments [20,21,22][20][21][22].
MICP could find many applications in the fields of water resources and, specifically, for hydrologic applications. Several of these applications, such as groundwater and soil decontamination, have already been suggested in existing literature. These could be classified based on the various underlying MICP mechanisms and the fundamental target for each application, as shown in Figure 2. The classification includes four groups: (i) groundwater and soil remediation, (ii) generation of a low hydraulic conductivity barrier for inhibiting fluid transport, (iii) gaining cohesion to inhibit particles movement along with fluids, and (iv) for generation of porous media of controlled mechanical and hydraulic properties that could be used in fluid flow studies. The first application involves MICP pathways for remediation while the second and third applications are based on the hydraulic and mechanical properties alteration via MICP. Finally, the fourth application is routed on the combination of hydraulic and mechanical properties alteration.
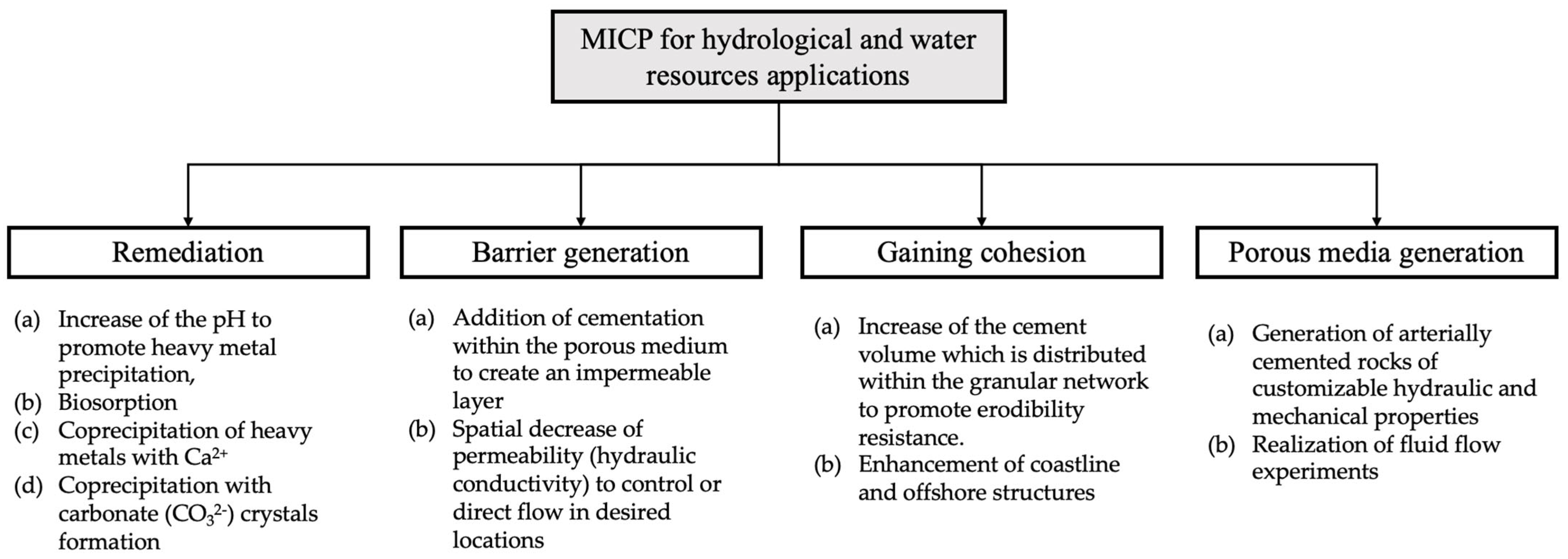
Figure 2.
The MICP underlying mechanisms and the fundamental target for each application.
2. Bioremediation via MICP
Bioremediation through MICP stands as a significant and promising approach in tackling environmental contamination challenges since it mimics natural processes, it is versatile as it can be applied to a wide range of contaminated environments, including soil and groundwater, it has minimum environmental footprint, and is less costly and energy-intensive compared to other remediation techniques (e.g., chemical precipitation, adsorption, ion exchange, and membrane separation). This section reviews the biocementation pathways of MICP which are the underlying principles behind it. That is, MICP can be used to solidify and immobilize contaminants, particularly toxic metals, through various precipitating mechanisms. It offers an effective means to transform hazardous pollutants into stable, less mobile forms, thus preventing their migration and potential harm to the environment and human health.
The main mechanism that relates to this group of applications (Figure 2—remediation) is the solidification of contaminants. This can be succeeded via the increase in the pH (typically achieved by the release of ammonia ions) to promote (i) heavy metal precipitation, (ii) biosorption, and either (iii) coprecipitation of heavy metals with Ca2+ or (iv) copre cip itation with carbonate (CO32−) crystals formation [23]. The four pathways might coexist. For example, Jiang et al. [24] suggested that Pb immobilization involves abiotic and biotic precipitation, as well as biosorption. The coexistence of various combinations of pathways has been reported in other studies also [25,26,27][25][26][27].
The toxic metal mineralization through MICP relies on ureolytic bacteria secreting urease to break down urea and the products are NH3 and CO2 (chemical Equation (3)). Following this, these substances reach a state of equilibrium within the solution, resulting in the creation of bicarbonate, ammonium, and hydroxide compounds (see Equations (4) and (5)) leading to an increase in both the alkalinity and carbonate levels. Finally, the formation of calcite is induced, with bacterial cells acting as the nuclei in an environment with high Ca2+ concentrations. In the presence of adequately active divalent cations, the carbonate has the potential to undergo precipitation from the solution and, therefore, toxic metals are mineralized by coprecipitation with carbonate (CO32−) crystals, as shown in chem ical Equation (6) [28]. The letter M in chemical Equation (6) denotes heavy metals (HMs).
(NH
(NH
2
)
2
CO + H
2
O → 2NH
3
+ CO
2
(3)
2NH3 + 2H2O → 2NH4+ + 2OH−
CO
CO2 + 2OH− → HCO3− + OH− → CO32− + H2O
O (5)
Ca2+ + CO32− → CaCO3 (↓) + M (HMs) → MCO3 (↓)
xM
2+
+ (1 − x)Ca
2+
+ CO
32−
→ Ca
(1−x)
M
x
CO
3 (s)
(s)
Throughout MICP and in the case of calcium sites substitution, heavy metals containing divalent ions like Cd2+, Zn2+, Pb2+, Cu2+, Co2+, Fe2+, and Ni2+ replace Ca2+ ions, leading to the formation of heavy metal carbonates [28,29,30,31,32][28][29][30][31][32]. This transformation effectively changes these heavy metals from a bioavailable state to a non-bioavailable form, rendering them less accessible to living organisms [31]. The effectiveness of ureolytically active bacteria used in MICP process in removing heavy metals is attributed to the similarity in their divalent ion formation with calcium (Ca2+), as well as the considerable surface area and abundance of negative ions on the cell surface.
The literature contains many MICP studies with successful outcomes in terms of immobilizing heavy metals via urea hydrolysis, including the listed heavy metals but also extent to other than divalent metals or radionuclides such as Cr6+, As3+, and 90Sr which can be precipitated to form their own insoluble carbonate minerals via the same route (see chemical Equation (6)) [27,33,34][27][33][34]. Alternatively, they have the potential to undergo coprecip itation with calcium carbonate (as depicted in chemical Equation (7)), provided that calcifying micro-organisms can survive in environments containing toxic metals. Those heavy metal ions that possess ion radii similar to that of Ca2+ (approximately 1.0 Å) have the capability to integrate into the crystal lattice of CaCO3 (adsorption in the intercellular spaces of CaCO3—biosorption) [35]. This integration can occur through processes like isomorphic substitution, where the metal ions replace calcium ions, or by penetrating the interstice or defect of crystal. Subsequently, these heavy metals undergo a transformation from soluble ions into forms that are not soluble, effectively preventing their re-release into the environment.
As stated previously, there are several examples of the success of contaminants removal demonstrating the applicability of the fundamentals of the method. For example, 95% removal of Cr and Pb was reported in farmland soil via the use of urease-producing fungi in just 12 days [33] and a 90% removal of multiple heavy metals (nickel, lead, copper, cobalt, zinc, and cadmium) in aqueous solution was achieved in the study by Li et al. [36]. The removal efficiencies reported for lead and chromium by He et al. [27] were as high as 86% and 76.8%, respectively, when the initial metal concentration was 25 mg/L. The use of native ureolytic bacteria for 90Sr in groundwater was also proven successful [37] with a removal rate of 59% when S. pasteurii was used in two-dimensional porous media reactors [38], while the use of mixed extracted ureolytic bacteria from mining sites was proven to be 83% successful [25]. In a study by Chen et al. [39], S. pasteurii was employed for the remediation of soil contaminated with Pb. The findings indicated a substantial reduction of 76.34% in the leaching of heavy metals from the soil subsequent to the remediation process. Mwandira et al. [40] reached 100% removal of high concentrations of Pb2+ via MICP. Peng et al. [29] successfully removed 99.50% of Cd within 7 days. Using indigenous bacterial, the biostimulation technique employed to enhance the MICP rates for copper immobilization resulted in notable decrease in the exchangeable soluble copper fraction in the soil, dropping from an initial level of 45.54 mg/kg to 1.55 mg/kg [41].
The success of the method depends on several factors. In most of the published studies, it is quantified as the efficiency of removing heavy metals expressed as a percentage of removal. One inhibiting factor is the bacterial toxicity which is a critical factor for contaminant removal [42,43][42][43]. The toxicity due to the presence of heavy metals is known to typically inhibit microbial activity, which, in turn, slows the bioremediation procedure, resulting in lower efficiency. Qiao et al. [43] studied the toxicity effects of these heavy metals to bacteria used in MICP, reporting that those effects, from most to least toxic, were cadmium, zinc, nickel, and copper. The bacterial strains, their population and urease activity, the calcium source and concentration of chemicals, the pH and initial concentration of the contaminant, and the type of the contaminant are some of the factors that need to be taken into account when designing such a remediation scheme [24,29,43,44][24][29][43][44]. Such factors interact with one another but also interact with the soil properties [43,44][43][44] and, therefore, to this point, laboratory experiments are required to simulate field conditions before applying the process in the field. For example, MICP was proven not suitable for very fine-grained soils (<100 μm), such as mine tailings [45]. Bacterial strains capable of establishing an alkaline nature through metabolism and, subsequently, achieving it are key to the process. At the same time, there is a clear link between the precipitation of carbonate and the extent of urea hydrolysis, controlled by the bacterial urease enzyme. Urease catalyzes the conversion of urea into products at a rate significantly higher than the spontaneous decomposition rate [35]. As a result, the bacterial population and levels of urease activity play an important role in bioremediation. It is important to allow enough time for reactions to occur, but, also, the treatment cycles should not last long as the urease activity is known to decline with time [46]. In addition, there is an interplay between reaction times and treatment duration as the two should be compatible. Bacterial strains should be also carefully selected based on the information known about the contaminant (type and concentration) due to its toxicity effects on micro-organisms, while in cases where the pH indicates acidic environment, it should be regulated so that it remains alkaline. The calcium source and the chemicals’ concentration clearly depend on the characteristics and nature of the contaminant.
Other challenges related to the application of MICP for remediation include the identification of the appropriate MICP recipe (mainly, urea mass) and the recovery of ammonium to improve the economic and environmental benefits [47]. In comparison to alternative methods, the remediation process involving MICP is linked to elevated expenses due to the costs of reactant materials and the uneven effects observed in large-scale fields. Additionally, there may be environmental implications associated with MICP, as it has the potential to disrupt ecological balance and might not adapt effectively to intricate environmental conditions [48]. On a practical level, the long-term performance of heavy metal removal has not been assessed up to this point and there is no picture on a complete and comprehensive life-cycle assessment [49].
MICP offers a significant advantage over conventional methods due to its ability to withstand redox-insensitive conditions, ensuring that heavy metal carbonates remain non-toxic, insoluble, and inaccessible, giving long-term stability. According to Gadd [50], MICP can overcome some limitations associated with biosorption. Heavy metals that have been precipitated are integrated into mineral crystals, rendering them stable in geological terms. Zhao et al. [49] demonstrated the stability of Pb-MICP precipitates under continuous acid degradation. Moreover, MICP provides a low-cost and eco-friendly method for heavy metal remediation through bioimmobilization [40]. In terms of solidifying heavy metal-contaminated soils, MICP contributes to better strength improvement while maintaining better hydraulic conductivity and higher durability, and it rarely damages the original soil structure during grouting, making it more environmentally friendly compared to other agglutinate binders [23,48][23][48].
One common problem for MICP applications is usually upscaling. However, in this direction there are multiple field experiments in the literature proving its viability and applicability to real environmental conditions and industrial use. Few of these studies include the work by Fujita et al. [37] applied at the Hanford 100-N Area in Washington.
3. Mechanical and Hydraulic Properties Alteration
The addition of cementation within the pore network and on the surface of the grains results in strength enhancement and hydraulic conductivity alteration. In most hydrological applications, the hydraulic conductivity is the main property that controls any design. This section presents the two properties that change when applying an MICP injection scheme with a focus on hydraulic conductivity which is most relevant to the examined applications. The analysis focuses on both the macro- and micro-scales since both the amount of cementation and its microstructure characteristics control the response and behaviour of the resulting products. The properties of the treated products are influenced by the characteristics of the base material (particle roughness, shape, size, and width of particle size distribution) as well as the distribution and structure of the cement within the medium (including the amount, crystal shape, size, and calcite location) [51,52,53,54,55][51][52][53][54][55]. Additionally, the cement properties are also governed by various other factors related to the MICP procedure and external/environmental factors, such as the number of injections, chemical concentration in each injection, retention time between injections, infiltration (or injection) rate, and bacterial density/activity, temperature, pH, and presence of oxygen [10,52,56][10][52][56]. Consequently, the mechanical properties may vary from one study to another, depending on the selection of biochemical parameters and the specific experimental protocol. To accurately assess the effects of each parameter, whether biological or related to the grains, it is essential to isolate other influences and follow a consistent procedure. Both the strength enhancement and the hydraulic conductivity heavily depend on the microstructure. Three distinct and ideal types of grain/cement structures have been identified [57], as shown in Figure 3. These are: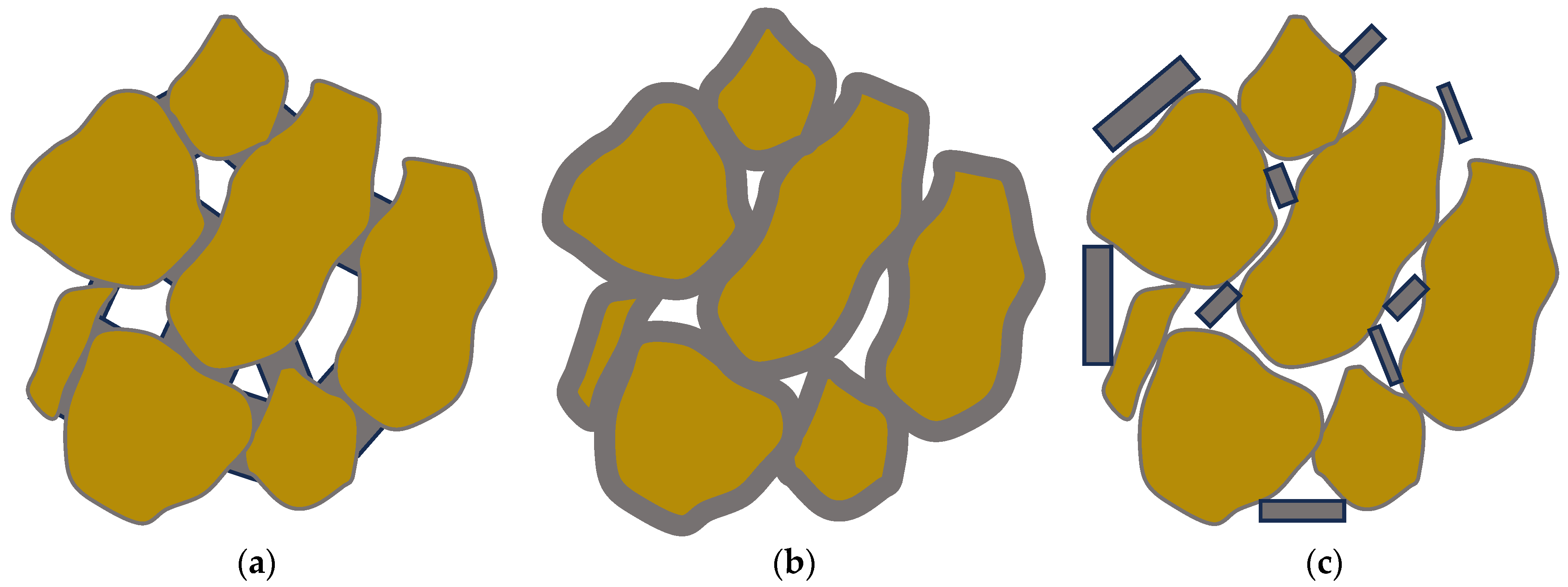
Figure 3.
The three idealized of grain/cement structures: (
a
) contact-cementing, (
b
) grain coating, and (
c
) matrix-supporting.
- (i)
-
contact-cementing, in which the carbonate crystals precipitate on and around the contacts between the grains (see schematic in Figure 3a);
- (ii)
-
grain coating, in which cement forms a uniform film around the grains (see schematic in Figure 3b); and
- (iii)
-
matrix-supporting, in which the precipitate is identified within the granular network (see schematic in Figure 3c).
3.1. Strength Enchancement
In the existing literature, the most common method used to evaluate the strength of biocemented sands is the unconfined compressive strength (UCS), although other measurements, such as the small strain shear stiffness, Gmax, tangent modulus [14[14][57][58],57,58], friction angle, cohesion derived from triaxial compressional tests [59[59][60][61],60,61], and even tensile strength [62[62][63],63], have been evaluated. Starting from the biochemical factors, the literature suggests that the bacterial strain selected, the population and the urease activity, the type of calcium source, the concentration of chemicals, and the treatment duration and frequency of injections, but also the injecting method, affect the precipitating carbonate crystals in terms of size, type, and distribution within the pore network which, in turn, affect the resulting microstructure and, therefore, strength enhancement, as shown in Figure 3. Previous research by Cheng et al. [53] indicates that lower urease activity can enhance particle bonding and increase unconfined compressive strength (UCS) due to slower formation of carbonate and more effective particle bridging (see Figure 3a). The findings of this study were strengthened by the study of Konstantinou et al. [46], in which a protocol was proposed on the basis of selecting bacteria with reduced urease activity as part of a low-concentration cementation solution during the MICP procedure which leads to balanced and slower reactions, thereby promoting consistent, reproducible outcomes and, eventually, leading to more efficient and better strength enhancement. However, Whiffin [9] reported significant variability in enzyme levels, suggesting that consistent urease production cannot be guaranteed even under conducive growth conditions. This variability of enzymatic activity could be either resolved by using bacteria strains of lower variability or native micro-organism isolates which could also minimize any environmental impacts or by following proposed methods to generate bacterial populations with the desired urease activity [46]. The authors [46] linked the differences in behaviour with the microscale in which it was observed that when the urease activity was higher, the carbonate crystals formed were of comparable size, exhibiting a cubic shape and uniform distribution on the grain surface. With decreasing urease activity, the crystals grew larger and tended to form clusters, appearing to be more efficient in creating bridges between the particles. These observations were particularly noticeable in higher bacterial populations and explain the better strength enhancement obtained. Various bacterial types have been also used, demonstrating the success of the procedure with almost any ureaseorganisms [19,38,53,64,65,66,67][19][38][53][64][65][66][67]. The bacterial population does also have effects on the size and structure of the carbonate distribution. Wang et al. [68,69,70][68][69][70] used a microfluidic chip to observe the various biochemical MICP parameters in response to the carbonate precipitation. An example of a microfluidic chip experiment is shown in Figure 4. The injection process of bacterial and chemical solutions, distribution of bacteria, precipitation, calcium carbonate crystal growth process, and its spatial distribution were studied. The findings indicate that a higher bacterial density results in a higher precipitation rate of CaCO3, with more crystals but with a smaller average crystal volume [71]. This finding indicates that a medium range of optical densities leads to more efficient bonding of particles and, thus, higher strength.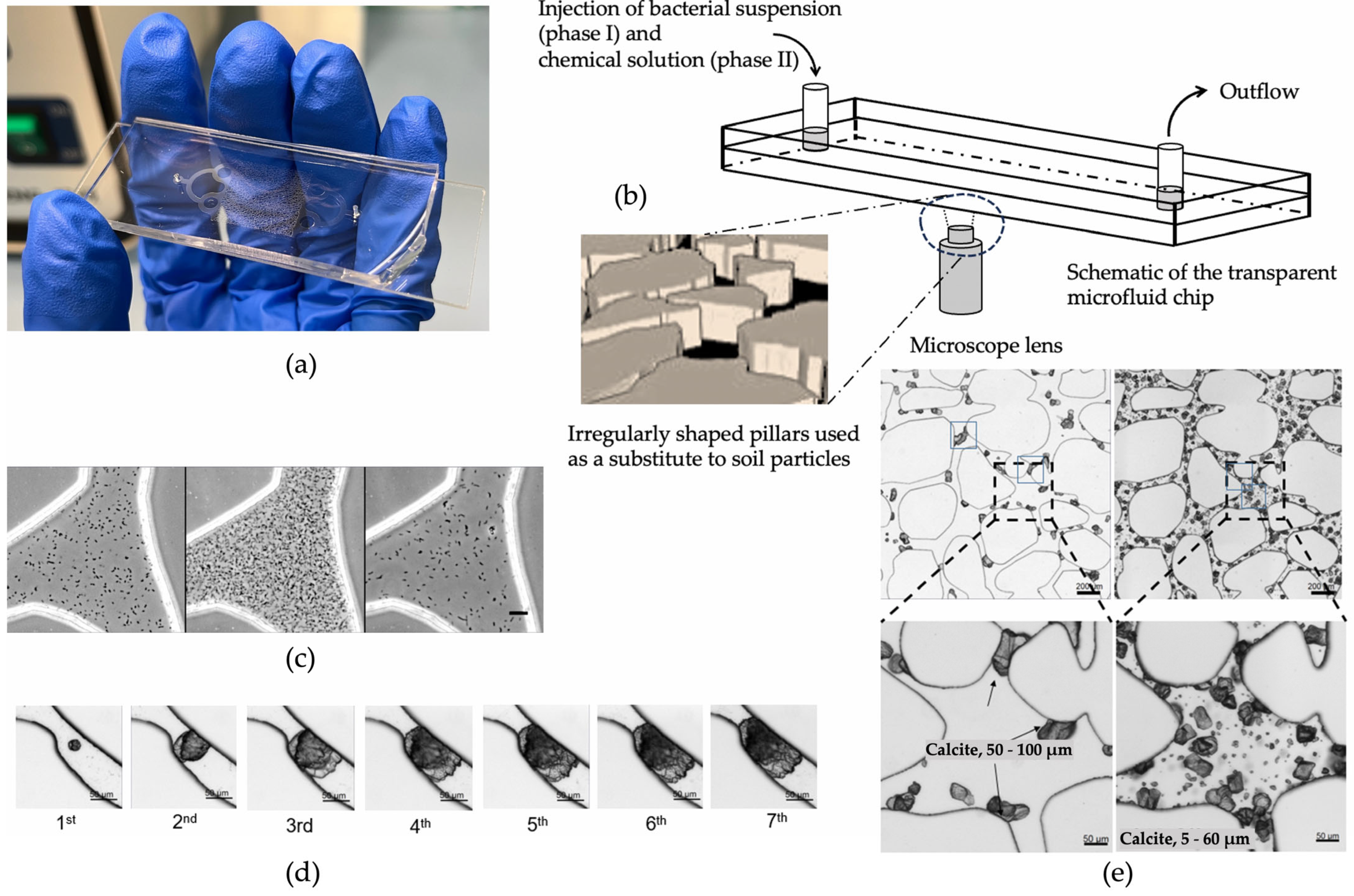 Figure 4. Schematic diagram of a MICP microfluidic example experiment [70]: (a) microfluidic chip; (b) schematic diagram of the injection process; (c) distribution of bacteria after injection, precipitation for 18 h, and injection of cementation solution; (d) calcium carbonate crystal growth process; (e) cementation of calcium carbonate in pores and throats.The amounts of chemicals are also important parameters for these investigations. Al Qabany and Soga [51] demonstrated that reducing the chemical concentration level increases the unconfined compressive strength of biotreated specimens. In a relevant study at the grain scale level [72], the extent of the strength enhancement of the MICP-treated specimens was linked to the differences in crystal sizes and numbers and their growth dynamics. The authors identified that, regardless of the concentration of cementation, decreasing the normalized input rate (treatment frequency and duration) of the cementation solution resulted in a substantial increase in UCS. A further reduction in the normalized input rate led to a slight increase in UCS values. Poisson’s ratio in MICP-treated sands was found to decrease with increasing calcium chloride concentration, indicating less lateral deformation and volume change at the same axial strain compared to lower concentrations [57]. The second group of factors is environmental factors. These could include factors such as the pH, the presence of oxygen, the salinity level, the saturation, the environmental temperature, the presence of competitive micro-organisms, applied stresses, etc. These, of course, have effects on both the gaining of cohesion but also on the hydraulic conductivity reduction. In the study by Cheng et al. [53], the unconfined compressive strength (UCS) was measured for various degrees of saturation. The authors found that lower degrees of saturation resulted in strength enhancement during the MICP procedure, likely due to more effective cementation at particle contacts (see Figure 3a). Triaxial testing revealed that the initial tangent Young’s modulus (Ei) of MICP-treated sands was controlled by the CaCO3 content and was less sensitive to an increase in effective confining pressure compared to untreated specimens [57]. Other studies also showed similar trends in friction angle and cohesion as a function of cementation level [59,60,61][59][60][61]. The durability of MICP-treated samples in freeze–thaw (FT) cycle tests was found to vary depending on the particle size distribution, with well-graded sands exhibiting improved resistance to FT cycles [53]. Additionally, conducting the MICP procedure at higher temperatures enhances the strength of the resulting samples [53,73,74][53][73][74]. However, at low or very high temperatures the bacterial growth was found to be lower, which has effects on crystal growth, as seen in the microfluidic experiments by Wang et al. [73] (see Figure 4). Such dynamics affect the resulting strength enhancement [75]. The third group of factors that affect the alteration of properties is granular network properties. The strength of cemented materials relies on the number of contact points and the position of the cement within the porous medium. A greater number of particles in a given volume leads to more contact points, distributing stresses evenly within the granular matrix and reducing stress on each particle. According to Konstantinou et al. [76], who systematically studied those effects, when cement is present at these contact points, it acts as a bonding agent, holding the particles together. The strength of these bonds depends on the amount of cementation around the contact points (effective cementation) which is shown in the ideal case in Figure 3a. In cases where there is no cementation, the contact points between particles become weak points. Large particles increase the likelihood of carbonate crystals forming on their surfaces, providing less effective cementation, resulting in a significant portion of the cementation not contributing much to strength improvement. While more contact points allow for more effective cementation, an excessive number of contact points requires a larger amount of cementation overall to enhance strength, as any uncemented contact points create preferential paths of failure under compression. Additionally, cementation may become random as the flow during treatment diverts from densely packed areas to those with less cementation. Very coarse sands with a larger average particle diameter had fewer particles but more surface area, making them less suitable for effective cementation, leading to consistently lower strength at any given cementation level (Figure 3b). On the other hand, very fine sands offered more contact points, making them better candidates for effective cementation, but their strength was still lower compared to fine sands [76,77][76][77]. If there are many contact points to be cemented, some might be left without cementation support, creating weak points susceptible to failure unless a substantial amount of cementation is provided. Fine-to-coarse sands strike a balance, providing the highest strength because of the combination of the absolute number of contact points and the ratio of contact points to surface grain area. An increase in the uniformity coefficient generates more contact points and reduces pore sizes, resulting in lower fluid flow during the treatment process. Therefore, mixtures with larger particles and a wide particle size distribution (PSD) achieved better strength enhancement since large particles offer fewer contact points, but the wider PSD reduces the chances of cementation depositing on the particle surfaces [76]. In materials with similar uniformity coefficients but different grain sizes, higher cementation levels were necessary to attain the same strength, primarily because smaller grains increase the likelihood of cementation occurring between particles rather than on their surfaces [76]. Particle shapes also influence mechanical properties [78]. Sub-rounded, spherical particles provided the highest strength due to their numerous contact points, while angular particles lacked sufficient contact points. Angular particles had initial strength from interlocking before cementation, and, while cementation increased strength, the effect was limited (i.e., diminishing returns with increasing cementation) [76]. On the other hand, Xiao et al. [78] demonstrated that a better base material for MICP treatments in terms of strength is provided by less round particles in mixtures of round and angular glass beads. Figure 5 presents unconfined compressive strength values with respect to cementation level based on the results of various studies [3,51,53,72,76,77,79,80,81][3][51][53][72][76][77][79][80][81]. The literature contains a large number of data on UCS, yet some studies have been chosen to demonstrate its correlation with cementation level. As seen in the graph, in which the y-axis is in a log scale, an exponential fit describes the correlations, showing that cementation level is a main contributing factor despite the fact that experimental conditions are different across the studies (grain characteristics, chemical concentrations, bacterial population, and urease activity, temperature, and saturation level). These conditions are reflected in the precipitation patterns at the microscale level.
Figure 4. Schematic diagram of a MICP microfluidic example experiment [70]: (a) microfluidic chip; (b) schematic diagram of the injection process; (c) distribution of bacteria after injection, precipitation for 18 h, and injection of cementation solution; (d) calcium carbonate crystal growth process; (e) cementation of calcium carbonate in pores and throats.The amounts of chemicals are also important parameters for these investigations. Al Qabany and Soga [51] demonstrated that reducing the chemical concentration level increases the unconfined compressive strength of biotreated specimens. In a relevant study at the grain scale level [72], the extent of the strength enhancement of the MICP-treated specimens was linked to the differences in crystal sizes and numbers and their growth dynamics. The authors identified that, regardless of the concentration of cementation, decreasing the normalized input rate (treatment frequency and duration) of the cementation solution resulted in a substantial increase in UCS. A further reduction in the normalized input rate led to a slight increase in UCS values. Poisson’s ratio in MICP-treated sands was found to decrease with increasing calcium chloride concentration, indicating less lateral deformation and volume change at the same axial strain compared to lower concentrations [57]. The second group of factors is environmental factors. These could include factors such as the pH, the presence of oxygen, the salinity level, the saturation, the environmental temperature, the presence of competitive micro-organisms, applied stresses, etc. These, of course, have effects on both the gaining of cohesion but also on the hydraulic conductivity reduction. In the study by Cheng et al. [53], the unconfined compressive strength (UCS) was measured for various degrees of saturation. The authors found that lower degrees of saturation resulted in strength enhancement during the MICP procedure, likely due to more effective cementation at particle contacts (see Figure 3a). Triaxial testing revealed that the initial tangent Young’s modulus (Ei) of MICP-treated sands was controlled by the CaCO3 content and was less sensitive to an increase in effective confining pressure compared to untreated specimens [57]. Other studies also showed similar trends in friction angle and cohesion as a function of cementation level [59,60,61][59][60][61]. The durability of MICP-treated samples in freeze–thaw (FT) cycle tests was found to vary depending on the particle size distribution, with well-graded sands exhibiting improved resistance to FT cycles [53]. Additionally, conducting the MICP procedure at higher temperatures enhances the strength of the resulting samples [53,73,74][53][73][74]. However, at low or very high temperatures the bacterial growth was found to be lower, which has effects on crystal growth, as seen in the microfluidic experiments by Wang et al. [73] (see Figure 4). Such dynamics affect the resulting strength enhancement [75]. The third group of factors that affect the alteration of properties is granular network properties. The strength of cemented materials relies on the number of contact points and the position of the cement within the porous medium. A greater number of particles in a given volume leads to more contact points, distributing stresses evenly within the granular matrix and reducing stress on each particle. According to Konstantinou et al. [76], who systematically studied those effects, when cement is present at these contact points, it acts as a bonding agent, holding the particles together. The strength of these bonds depends on the amount of cementation around the contact points (effective cementation) which is shown in the ideal case in Figure 3a. In cases where there is no cementation, the contact points between particles become weak points. Large particles increase the likelihood of carbonate crystals forming on their surfaces, providing less effective cementation, resulting in a significant portion of the cementation not contributing much to strength improvement. While more contact points allow for more effective cementation, an excessive number of contact points requires a larger amount of cementation overall to enhance strength, as any uncemented contact points create preferential paths of failure under compression. Additionally, cementation may become random as the flow during treatment diverts from densely packed areas to those with less cementation. Very coarse sands with a larger average particle diameter had fewer particles but more surface area, making them less suitable for effective cementation, leading to consistently lower strength at any given cementation level (Figure 3b). On the other hand, very fine sands offered more contact points, making them better candidates for effective cementation, but their strength was still lower compared to fine sands [76,77][76][77]. If there are many contact points to be cemented, some might be left without cementation support, creating weak points susceptible to failure unless a substantial amount of cementation is provided. Fine-to-coarse sands strike a balance, providing the highest strength because of the combination of the absolute number of contact points and the ratio of contact points to surface grain area. An increase in the uniformity coefficient generates more contact points and reduces pore sizes, resulting in lower fluid flow during the treatment process. Therefore, mixtures with larger particles and a wide particle size distribution (PSD) achieved better strength enhancement since large particles offer fewer contact points, but the wider PSD reduces the chances of cementation depositing on the particle surfaces [76]. In materials with similar uniformity coefficients but different grain sizes, higher cementation levels were necessary to attain the same strength, primarily because smaller grains increase the likelihood of cementation occurring between particles rather than on their surfaces [76]. Particle shapes also influence mechanical properties [78]. Sub-rounded, spherical particles provided the highest strength due to their numerous contact points, while angular particles lacked sufficient contact points. Angular particles had initial strength from interlocking before cementation, and, while cementation increased strength, the effect was limited (i.e., diminishing returns with increasing cementation) [76]. On the other hand, Xiao et al. [78] demonstrated that a better base material for MICP treatments in terms of strength is provided by less round particles in mixtures of round and angular glass beads. Figure 5 presents unconfined compressive strength values with respect to cementation level based on the results of various studies [3,51,53,72,76,77,79,80,81][3][51][53][72][76][77][79][80][81]. The literature contains a large number of data on UCS, yet some studies have been chosen to demonstrate its correlation with cementation level. As seen in the graph, in which the y-axis is in a log scale, an exponential fit describes the correlations, showing that cementation level is a main contributing factor despite the fact that experimental conditions are different across the studies (grain characteristics, chemical concentrations, bacterial population, and urease activity, temperature, and saturation level). These conditions are reflected in the precipitation patterns at the microscale level.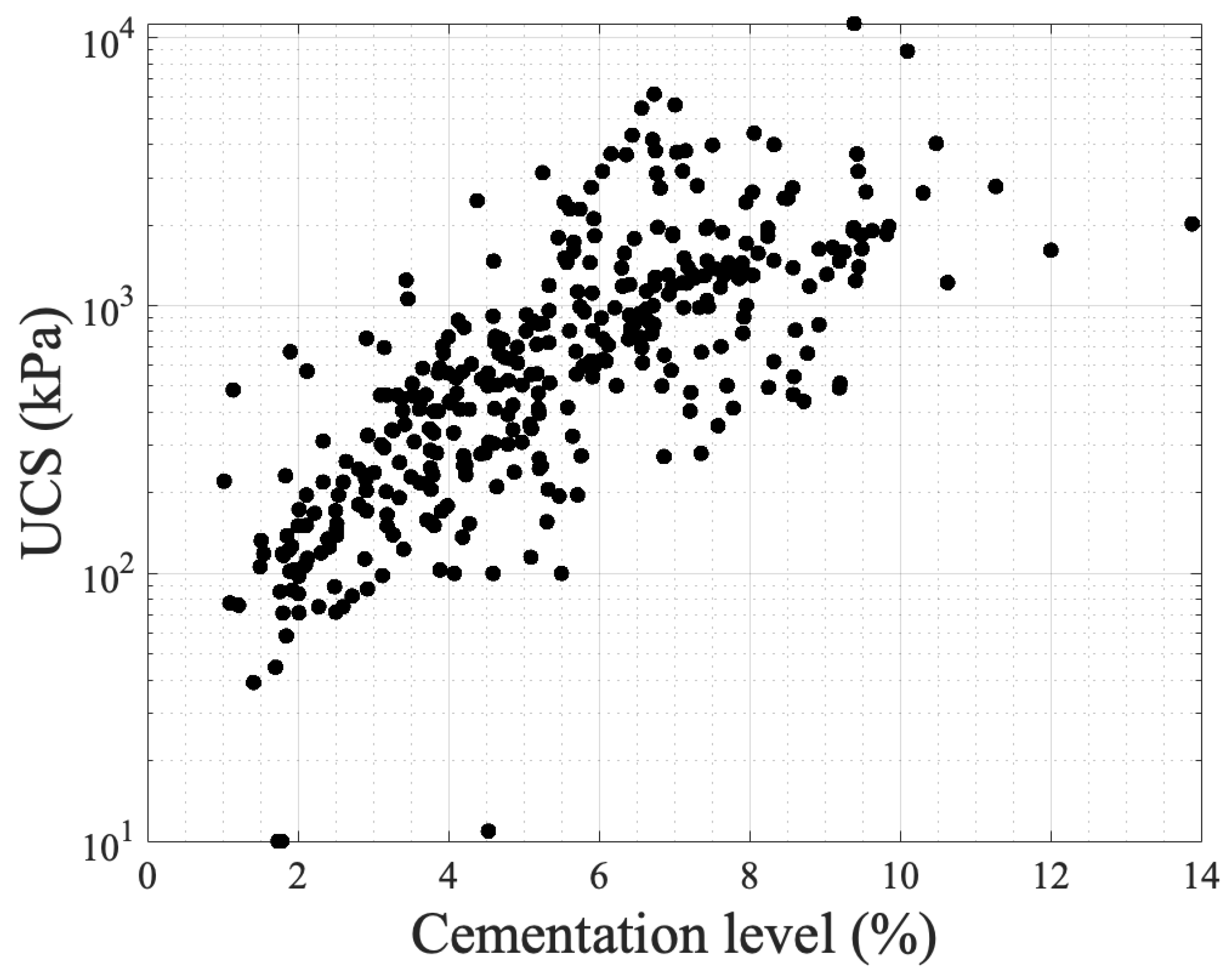 Figure 5.The experimental results of UCS with respect to cementation level as obtained in various studies.
Figure 5.The experimental results of UCS with respect to cementation level as obtained in various studies.3.2. Hydraulic Conductivity
Perhaps, the hydraulic conductivity is the property that is most understudied for MICP-treated porous media. This might be due to the very variable results and the fact that until this point most of studies focused on strength enhancement which is usually the main objective in geotechnical engineering studies. However, controlling the hydraulic conductivity in applications related to hydrology and water resources is of paramount importance. It is influenced by various factors related to the pore space, including its size, shape, and tortuosity (i.e., the Kozeny–Carman equation). As stated previously, the fate of calcium carbonate crystals within the granular medium depends on the biochemical MICP parameters and the environmental factors but also on the properties of the porous medium itself. Previous research on MICP-treated sands has consistently shown that the hydraulic conductivity decreases significantly as the cementation level increases even up to 10 orders of magnitude. This reduction in hydraulic conductivity was reported in several studies [51,76,79,82,83,84,85,86,87,88][51][76][79][82][83][84][85][86][87][88]. However, there was an exception noted in the study by Whiffin et al. [3], where the measured hydraulic conductivity showed little change after treatment. This was supported by other studies suggesting that specimens with lower amounts of precipitated calcite experience a less significant reduction in relative hydraulic conductivity [89]. In the studies by Dawoud et al. [80[80][90][91],90,91], the hydraulic conductivity was shown to have an initial ‘stable’ phase at the very low cementation region in which there was almost no reduction in hydraulic conductivity. Song et al. [92] presented a typical or normalized reduction profile as shown in Figure 6. In the initial stage of low cementations (stage I), the decrease in hydraulic conductivity occurs at a gradual pace and is marginal. Subsequently, there is a rapid decline in hydraulic conductivity (stage II) at moderate cementation levels. Finally, at high cementations, hydraulic conductivity reaches a nearly constant level which is very low (stage III). Considering this diagram, the findings of the previous studies could be combined and interpreted given that in the study by Whiffin et al. [3], the cementation levels were closer to the lower cementation levels compared to the rest. The general sense, though, is based on a comparison between hydraulic conductivity and UCS for Portland cemented samples and MICP-treated samples, that there is a smaller trade-off between hydraulic conductivity and strength in the case of MICP, suggesting a relative ability of retaining soil hydraulic conductivity [79].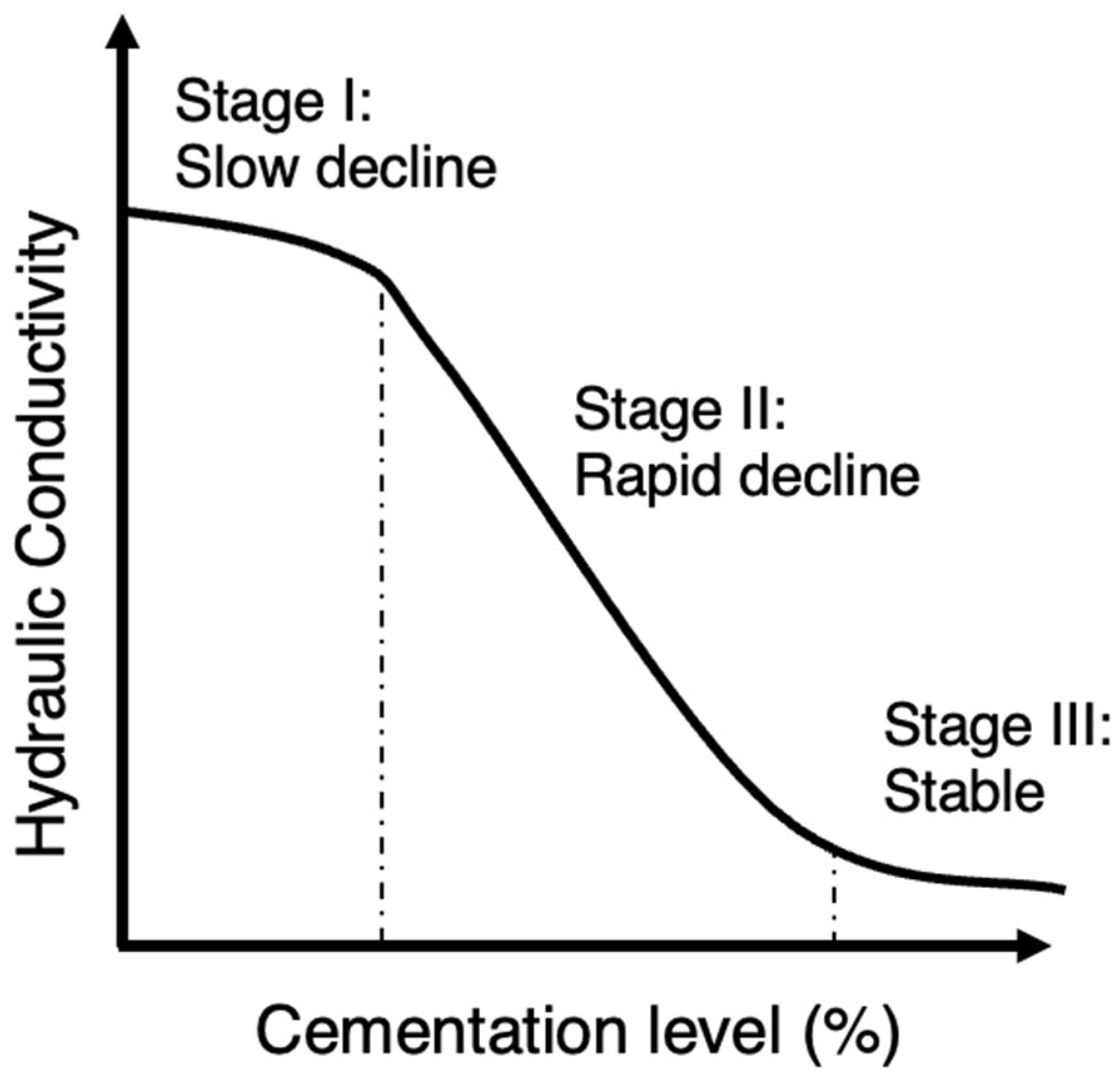 Figure 6.Beyond this general profile, which only presents a correlation with the overall volume of cement added, the resulting hydraulic conductivity could be explained to a great extent by evaluating the microscale response. This involves assessing properties such as carbonate crystal type, distribution, and size, which are routed on the three groups of factors mentioned earlier. The level of urease activity employed during treatment had only a minor effect on hydraulic conductivity reduction in the study by Cheng et al. [53] and according to Choi et al. [93]. However, in the research by Konstantinou et al. [46], the higher the urease activity was, the more clogged the specimen was at the injection point, decreasing dramatically the flow rate in subsequent injections. The difference between the two studies might be attributed to the fact that in the second work, the range of urease activities was wider. Also, a lower hydraulic conductivity was measured for higher bacterial populations [94]. Al Qabany and Soga [51] reported that the high concentration cementation solution produced a quicker and greater reduction in the coefficient of hydraulic conductivity, suggesting that higher concentrations of calcium chloride and urea lead to larger calcite crystals and a more uniform distribution of precipitation when using lower concentrations. The larger crystals can cause early clogging, as observed in their study. Similar findings were reported by other studies [91,95][91][95]. In the study by Duo et al. [95], the hydraulic conductivity gradually decreased as the concentration of the solidification solution increased, with a maximum reduction of approximately three orders of magnitude. The hydraulic conductivity coefficient reduction was particularly prominent up to a concentration of 1.5 mol/L, beyond which it remained constant despite having higher concentrations of chemicals. The authors linked this behaviour with the microstructure stating that during biocementation, calcium carbonate accumulated on the surface of the sand particles (see Figure 3b) and filled the gaps between them, leading to a gradual reduction in the volume of sand pores and, subsequently, a decrease in hydraulic conductivity. According to the authors, these findings of absolute hydraulic conductivity values suggest that the MICP technique holds promise for seepage control in pond and landfill engineering projects in desert areas due to the low hydraulic conductivity obtained [95]. Dawoud et al. [91] classified the hydraulic conductivity reduction in three phases: during the initial stages of treatment hydraulic conductivity shows a slight decrease or remains relatively unchanged. At this point, a small amount of precipitated calcite adds stiffness to the soil without causing pore clogging. This phase can be represented by a linear relationship with a slight negative slope (in agreement with the profile in Figure 6). As the precipitation of calcite continues, a certain threshold is reached where the accumulated calcite starts to clog the pores, leading to a new steeper trend of decreasing hydraulic conductivity after each treatment. During this stage, the concentration of the chemical solutions used in the treatment significantly influences the soil’s behavior and characteristics (again in agreement with Figure 6). The study noticed that using a 1M concentration for the urea–CaCl2 solution resulted in an earlier transition to the clogging phase. Once clogging initiates, the distribution of MICP becomes more uncertain. Blocked flow paths cause new precipitates to accumulate near the injection point, resulting in less uniform treatment across the sample. This phenomenon is consistent with the findings of Qabany and Soga [51]. The calcium source is also another biochemical factor that has effects on hydraulic conductivity. In the study by Kadhim et al. [96], the incorporation of a cementation solution containing calcium chloride derived from eggshells had a substantial impact on hydraulic conductivity. However, the effect was more pronounced in the silica sand samples rather than the river sands as in the former higher hydraulic conductivity reduction was observed. In other studies, the use of calcium acetate caused the greatest hydraulic conductivity reduction followed by calcium chloride. A very low hydraulic conductivity reduction was observed with the use of calcium nitrate [97]. MICP process under lower saturation conditions is more favorable if the goal is to improve the mechanical properties while still maintaining relatively high residual hydraulic conductivity [79]; however, it was also reported that there is a general trend of decreasing hydraulic conductivity with the increase in produced calcite content (CaCO3) irrespective of the degree of saturation at which the soil was treated [98]. The differences observed are likely due to the interaction effects (a variable is behaving differently at various levels of another variable) added from the choice of other biochemical parameters. The relative density (RD) and injection volume in a single injection event (VIP—void injection percentage) were also studied showing a negative correlation with hydraulic conductivity [99]. That is, as the two factors increased, there was a corresponding decrease in hydraulic conductivity. This reduction in hydraulic conductivity was attributed to several reasons. With an increase in RD, the pore volume (PV) decreased, leading to the formation of smaller and less permeable pore throats. However, at very high RD some inconsistency was observed, likely due to the pore throats becoming smaller, increasing the likelihood of clogging and facilitating the creation of preferential flow paths during bacterial suspension and cementation fluid injection. Moreover, the decrease in hydraulic conductivity with increasing VIP was attributed to localized bacterial concentration near the injection point, which promoted more significant calcite precipitation in that specific region. This localized effect led to a reduction in hydraulic conductivity in the surrounding area [99]. One of the very few environmental factors studied for MICP in relation to the reduction of hydraulic conductivity was the use of seawater. The hydraulic conductivity coefficients of the samples treated with natural seawater-based biocementation were slightly higher compared to those treated with freshwater-based biocementation. This difference was attributed to a higher precipitation of carbonates in the freshwater columns than in the seawater columns. However, despite this disparity, using seawater-based biocementation instead of freshwater cementation did not have a significant impact on hydraulic conductivity [22]. Similarly, the ureolytic bacteria utilized by Cheng et al. [67] were acclimated to high-salinity conditions by employing a growth medium containing high concentrations of ammonium sulfate ((NH4)2SO4) to help prevent significant osmotic effects when exposed to seawater. The use of seawater supplemented with urea, instead of a concentrated cementation solution, had no substantial impact on the hydraulic conductivity per carbonate formed. However, the same carbonate formation resulted in higher strength in the seawater cementation trials, which, consequently, allowed for greater hydraulic conductivity for a given level of strength [67]. In terms of injection strategy, the use of continuous flow technique resulted in a significant reduction in hydraulic conductivity compared to the stopped-flow technique. This was attributed to the higher likelihood of columns treated with continuous flow to experience plugging near the injection source [84]. Also, the presence of a stationary liquid during biocementation resulted in slightly higher hydraulic conductivity reduction [100]. The work by Konstantinou et al. [76,82][76][82] on the grain characteristics effects on hydraulic conductivity revealed valuable insights on this link. Hydraulic conductivity undergoes a significant decrease when cement is present at contact points, leading to a reduction in pore throat size. Materials with numerous particle-to-particle contacts prove challenging to decrease hydraulic conductivity, as many contacts require cementing. Such materials regulate flow through paths with larger pores and pore throats, which involve uncemented particle contacts. As the grain size increases in a packed bed of solids, the hydraulic conductivity is expected to rise as well (see example MicroCT images in Figure 7a–c). For example, the Kozeny–Carman equation incorporates the squared average particle diameter in its empirical equation’s numerator. This trend of reduced hydraulic conductivity with increased cementation was also observed in very fine to coarse sands during the study and is in agreement with other studies where a D10 increase, resulted in a smaller reduction in relative hydraulic conductivity [89].The typical reduction profile of hydraulic conductivity with respect to cementation level.
Figure 6.Beyond this general profile, which only presents a correlation with the overall volume of cement added, the resulting hydraulic conductivity could be explained to a great extent by evaluating the microscale response. This involves assessing properties such as carbonate crystal type, distribution, and size, which are routed on the three groups of factors mentioned earlier. The level of urease activity employed during treatment had only a minor effect on hydraulic conductivity reduction in the study by Cheng et al. [53] and according to Choi et al. [93]. However, in the research by Konstantinou et al. [46], the higher the urease activity was, the more clogged the specimen was at the injection point, decreasing dramatically the flow rate in subsequent injections. The difference between the two studies might be attributed to the fact that in the second work, the range of urease activities was wider. Also, a lower hydraulic conductivity was measured for higher bacterial populations [94]. Al Qabany and Soga [51] reported that the high concentration cementation solution produced a quicker and greater reduction in the coefficient of hydraulic conductivity, suggesting that higher concentrations of calcium chloride and urea lead to larger calcite crystals and a more uniform distribution of precipitation when using lower concentrations. The larger crystals can cause early clogging, as observed in their study. Similar findings were reported by other studies [91,95][91][95]. In the study by Duo et al. [95], the hydraulic conductivity gradually decreased as the concentration of the solidification solution increased, with a maximum reduction of approximately three orders of magnitude. The hydraulic conductivity coefficient reduction was particularly prominent up to a concentration of 1.5 mol/L, beyond which it remained constant despite having higher concentrations of chemicals. The authors linked this behaviour with the microstructure stating that during biocementation, calcium carbonate accumulated on the surface of the sand particles (see Figure 3b) and filled the gaps between them, leading to a gradual reduction in the volume of sand pores and, subsequently, a decrease in hydraulic conductivity. According to the authors, these findings of absolute hydraulic conductivity values suggest that the MICP technique holds promise for seepage control in pond and landfill engineering projects in desert areas due to the low hydraulic conductivity obtained [95]. Dawoud et al. [91] classified the hydraulic conductivity reduction in three phases: during the initial stages of treatment hydraulic conductivity shows a slight decrease or remains relatively unchanged. At this point, a small amount of precipitated calcite adds stiffness to the soil without causing pore clogging. This phase can be represented by a linear relationship with a slight negative slope (in agreement with the profile in Figure 6). As the precipitation of calcite continues, a certain threshold is reached where the accumulated calcite starts to clog the pores, leading to a new steeper trend of decreasing hydraulic conductivity after each treatment. During this stage, the concentration of the chemical solutions used in the treatment significantly influences the soil’s behavior and characteristics (again in agreement with Figure 6). The study noticed that using a 1M concentration for the urea–CaCl2 solution resulted in an earlier transition to the clogging phase. Once clogging initiates, the distribution of MICP becomes more uncertain. Blocked flow paths cause new precipitates to accumulate near the injection point, resulting in less uniform treatment across the sample. This phenomenon is consistent with the findings of Qabany and Soga [51]. The calcium source is also another biochemical factor that has effects on hydraulic conductivity. In the study by Kadhim et al. [96], the incorporation of a cementation solution containing calcium chloride derived from eggshells had a substantial impact on hydraulic conductivity. However, the effect was more pronounced in the silica sand samples rather than the river sands as in the former higher hydraulic conductivity reduction was observed. In other studies, the use of calcium acetate caused the greatest hydraulic conductivity reduction followed by calcium chloride. A very low hydraulic conductivity reduction was observed with the use of calcium nitrate [97]. MICP process under lower saturation conditions is more favorable if the goal is to improve the mechanical properties while still maintaining relatively high residual hydraulic conductivity [79]; however, it was also reported that there is a general trend of decreasing hydraulic conductivity with the increase in produced calcite content (CaCO3) irrespective of the degree of saturation at which the soil was treated [98]. The differences observed are likely due to the interaction effects (a variable is behaving differently at various levels of another variable) added from the choice of other biochemical parameters. The relative density (RD) and injection volume in a single injection event (VIP—void injection percentage) were also studied showing a negative correlation with hydraulic conductivity [99]. That is, as the two factors increased, there was a corresponding decrease in hydraulic conductivity. This reduction in hydraulic conductivity was attributed to several reasons. With an increase in RD, the pore volume (PV) decreased, leading to the formation of smaller and less permeable pore throats. However, at very high RD some inconsistency was observed, likely due to the pore throats becoming smaller, increasing the likelihood of clogging and facilitating the creation of preferential flow paths during bacterial suspension and cementation fluid injection. Moreover, the decrease in hydraulic conductivity with increasing VIP was attributed to localized bacterial concentration near the injection point, which promoted more significant calcite precipitation in that specific region. This localized effect led to a reduction in hydraulic conductivity in the surrounding area [99]. One of the very few environmental factors studied for MICP in relation to the reduction of hydraulic conductivity was the use of seawater. The hydraulic conductivity coefficients of the samples treated with natural seawater-based biocementation were slightly higher compared to those treated with freshwater-based biocementation. This difference was attributed to a higher precipitation of carbonates in the freshwater columns than in the seawater columns. However, despite this disparity, using seawater-based biocementation instead of freshwater cementation did not have a significant impact on hydraulic conductivity [22]. Similarly, the ureolytic bacteria utilized by Cheng et al. [67] were acclimated to high-salinity conditions by employing a growth medium containing high concentrations of ammonium sulfate ((NH4)2SO4) to help prevent significant osmotic effects when exposed to seawater. The use of seawater supplemented with urea, instead of a concentrated cementation solution, had no substantial impact on the hydraulic conductivity per carbonate formed. However, the same carbonate formation resulted in higher strength in the seawater cementation trials, which, consequently, allowed for greater hydraulic conductivity for a given level of strength [67]. In terms of injection strategy, the use of continuous flow technique resulted in a significant reduction in hydraulic conductivity compared to the stopped-flow technique. This was attributed to the higher likelihood of columns treated with continuous flow to experience plugging near the injection source [84]. Also, the presence of a stationary liquid during biocementation resulted in slightly higher hydraulic conductivity reduction [100]. The work by Konstantinou et al. [76,82][76][82] on the grain characteristics effects on hydraulic conductivity revealed valuable insights on this link. Hydraulic conductivity undergoes a significant decrease when cement is present at contact points, leading to a reduction in pore throat size. Materials with numerous particle-to-particle contacts prove challenging to decrease hydraulic conductivity, as many contacts require cementing. Such materials regulate flow through paths with larger pores and pore throats, which involve uncemented particle contacts. As the grain size increases in a packed bed of solids, the hydraulic conductivity is expected to rise as well (see example MicroCT images in Figure 7a–c). For example, the Kozeny–Carman equation incorporates the squared average particle diameter in its empirical equation’s numerator. This trend of reduced hydraulic conductivity with increased cementation was also observed in very fine to coarse sands during the study and is in agreement with other studies where a D10 increase, resulted in a smaller reduction in relative hydraulic conductivity [89].The typical reduction profile of hydraulic conductivity with respect to cementation level.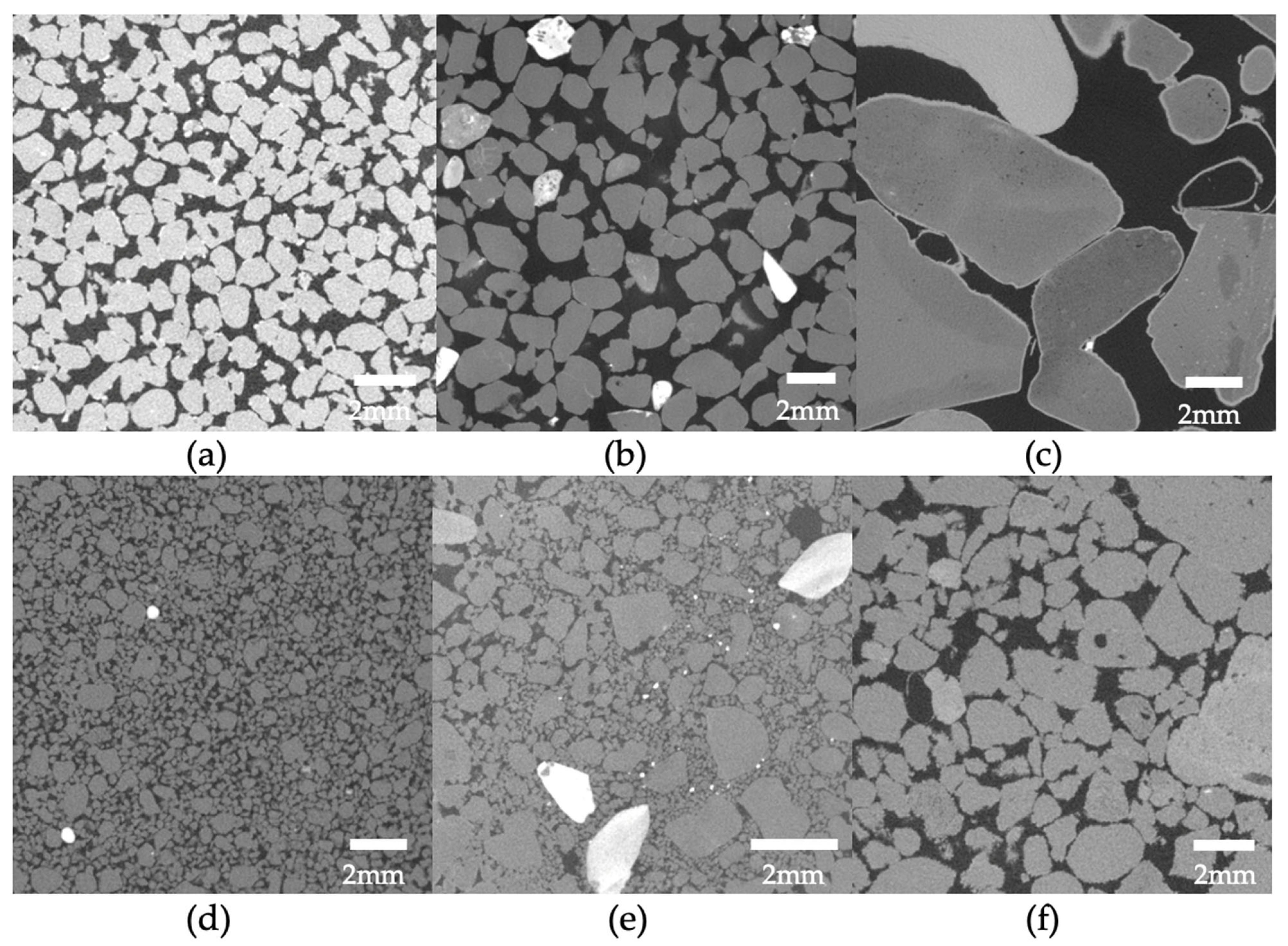 Figure 7. Example MicroCT images of biotreated sands by Konstantinou et al. [76]: (a–c) increasing grain size and (d–f) increasing grain size and width of particle size distribution.However, very coarse sands and gravel did not follow the same pattern and displayed a less controlled reduction in hydraulic conductivity [76]. In very coarse materials, carbonate precipitation mainly occurs on the surface of particles rather than at contacts among them. As a result, the reduction in hydraulic conductivity is not expected to be significant. Nevertheless, the lower number of contact points leads to fewer flow path options, explaining the rapid decline of hydraulic conductivity in very coarse particles at lower cementation levels. When comparing gravel and very coarse sand (both deemed ineffective for MICP treatment), gravel exhibited a larger reduction in hydraulic conductivity due to the lower number of available flow paths. Gravel’s erratic pore distribution and randomness, caused by its very large grains, provide for unpredictable fluid flow paths, resembling a system with small and few large pipes dominating the flow [76]. Hydraulic conductivity in relation to cementation is lower when the base material has a wider spread of particle size distribution (PSD) due to the narrower initial pore space distribution before cementation (see example MicroCT images in Figure 7d–f). The reduction in hydraulic conductivity with increasing carbonate content is also lower compared to more uniform sands, as there are too many narrow flow paths to be cemented (and, essentially, closed) when a higher number of contact points exists. This holds true for granular materials with wide PSD [76]. This was confirmed by other studies in which the hydraulic conductivity of cemented sand was found to be influenced also by its grain gradation in a similar manner. The reduction in the hydraulic conductivity coefficient during each MICP treatment cycle increased with higher values of the uniformity coefficient (Cu) and the curvature coefficient (Cc) [86]. On the other hand, the absolute value and reduction of hydraulic conductivity in two materials with similar uniformity coefficients but different grain sizes are the same, demonstrating that the dominant factor controlling flow is the spread of PSD [76]. Although the material with smaller grain sizes has more contact points, it provides more flow path options compared to the one with larger grain sizes. At the same time, the latter has a lower ratio of contact points over surface grain area, resulting in some of the cementation being consumed on the surface of the grains, leading to a lower rate of hydraulic conductivity reduction. While particle sphericity is known to impede flow, according to the Kozeny–Carman equation, significant hydraulic conductivity differences were only observed in the case of angular sand in the study by Konstantinou et al. [76]. However, the reduction trend with respect to cementation levels was similar for angular sand, fine and coarse glass beads, and fine and very coarse subrounded sand, indicating that grain size is the dominant factor in these cases. The flow paths are affected, to some extent, by grain shapes, but the addition of cementation causes proportional hydraulic conductivity reduction in spheres, sub-rounded, and angular sands. In the study by Song et al. [101], though, the non-spherical particles (crushed Ottawa sand) experienced the highest drop in hydraulic conductivity compared to the spherical particles despite having a lower calcium carbonate content showing a specific trend: the angular grains exhibited the highest hydraulic conductivity reduction, followed by the near-spherical particles, with the spherical particles showing the least reduction [101]. To investigate how pore-scale CaCO3 distributions affect the hydraulic conductivity of MICP-treated sands, the researchers used the Panda–Lake model [102]. This model incorporated three reduction factors: the porosity reduction factor, tortuosity reduction factor, and specific surface area reduction factor. Additionally, the Kozeny–Carman model was used to estimate hydraulic conductivity reduction with CaCO3 content, considering only the reduction of porosity [103]. By comparing the calculated hydraulic conductivity using the Panda–Lake and Kozeny–Carman models with the measured hydraulic conductivity reported in existing literature, the researchers developed an analytical model that can reasonably predict the hydraulic conductivity of MICP-treated sands for different CaCO3 contents and types of sands [103]. Lin et al. [103] performed an analysis on measured hydraulic conductivity values across various studies identifying that the grain coating (Figure 3b) Panda–Lake model provides reasonable fits to the data provided that the main mechanism is matrix-supporting. The Panda–Lake model takes into account, the shape, tortuosity, specific surface area (surface area of the grain/the volume of the grain), the statistical characteristics of the particle size distribution, the cement saturation of the pore space, the fraction of CaCO3 volume to the total volume of solids, and the specific surface area of the CaCO3 crystals. The matrix-supporting environment seen in Figure 3c shows smaller reduction of hydraulic conductivity with respect to cementation level. It follows, based on the findings of Lin et al. [103] and Konstantinou et al. [76], that the Kozeny–Carman equation would give better estimations of the reduction of hydraulic conductivity for the contact-cementing model. Even though the findings are in good agreement, there is room for further research to examine the combinations of factors and their effects on the resulting hydraulic conductivity. Figure 8 presents the data concentrated from the available references that measured hydraulic conductivity [3,22,51,53,67,76,79,82,83,84,85,86,87,88,89,91,92,93,94,95,96,97,98,99,100,101,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130][3][22][51][53][67][76][79][82][83][84][85][86][87][88][89][91][92][93][94][95][96][97][98][99][100][101][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130]. The y-axis is in a log scale. Despite the fact that there is a trend in reduction similar to the profile presented in Figure 5, the results are scattered showing a weak correlation with the cementation level. This is because not only the volume of cementation is required to identify the reduction in hydraulic conductivity but also the carbonate crystal size and distribution is required. Also, the initial configuration of the granular network is required, and this is the reason the Panda–Lake model performs better compared to the Kozeny–Carman equation. The results also show that UCS has a higher correlation coefficient (0.55) (Figure 5) compared to hydraulic conductivity (with a value of 0.1) showing more dependence on cementation level and less dependence on the granular and cement configuration.
Figure 7. Example MicroCT images of biotreated sands by Konstantinou et al. [76]: (a–c) increasing grain size and (d–f) increasing grain size and width of particle size distribution.However, very coarse sands and gravel did not follow the same pattern and displayed a less controlled reduction in hydraulic conductivity [76]. In very coarse materials, carbonate precipitation mainly occurs on the surface of particles rather than at contacts among them. As a result, the reduction in hydraulic conductivity is not expected to be significant. Nevertheless, the lower number of contact points leads to fewer flow path options, explaining the rapid decline of hydraulic conductivity in very coarse particles at lower cementation levels. When comparing gravel and very coarse sand (both deemed ineffective for MICP treatment), gravel exhibited a larger reduction in hydraulic conductivity due to the lower number of available flow paths. Gravel’s erratic pore distribution and randomness, caused by its very large grains, provide for unpredictable fluid flow paths, resembling a system with small and few large pipes dominating the flow [76]. Hydraulic conductivity in relation to cementation is lower when the base material has a wider spread of particle size distribution (PSD) due to the narrower initial pore space distribution before cementation (see example MicroCT images in Figure 7d–f). The reduction in hydraulic conductivity with increasing carbonate content is also lower compared to more uniform sands, as there are too many narrow flow paths to be cemented (and, essentially, closed) when a higher number of contact points exists. This holds true for granular materials with wide PSD [76]. This was confirmed by other studies in which the hydraulic conductivity of cemented sand was found to be influenced also by its grain gradation in a similar manner. The reduction in the hydraulic conductivity coefficient during each MICP treatment cycle increased with higher values of the uniformity coefficient (Cu) and the curvature coefficient (Cc) [86]. On the other hand, the absolute value and reduction of hydraulic conductivity in two materials with similar uniformity coefficients but different grain sizes are the same, demonstrating that the dominant factor controlling flow is the spread of PSD [76]. Although the material with smaller grain sizes has more contact points, it provides more flow path options compared to the one with larger grain sizes. At the same time, the latter has a lower ratio of contact points over surface grain area, resulting in some of the cementation being consumed on the surface of the grains, leading to a lower rate of hydraulic conductivity reduction. While particle sphericity is known to impede flow, according to the Kozeny–Carman equation, significant hydraulic conductivity differences were only observed in the case of angular sand in the study by Konstantinou et al. [76]. However, the reduction trend with respect to cementation levels was similar for angular sand, fine and coarse glass beads, and fine and very coarse subrounded sand, indicating that grain size is the dominant factor in these cases. The flow paths are affected, to some extent, by grain shapes, but the addition of cementation causes proportional hydraulic conductivity reduction in spheres, sub-rounded, and angular sands. In the study by Song et al. [101], though, the non-spherical particles (crushed Ottawa sand) experienced the highest drop in hydraulic conductivity compared to the spherical particles despite having a lower calcium carbonate content showing a specific trend: the angular grains exhibited the highest hydraulic conductivity reduction, followed by the near-spherical particles, with the spherical particles showing the least reduction [101]. To investigate how pore-scale CaCO3 distributions affect the hydraulic conductivity of MICP-treated sands, the researchers used the Panda–Lake model [102]. This model incorporated three reduction factors: the porosity reduction factor, tortuosity reduction factor, and specific surface area reduction factor. Additionally, the Kozeny–Carman model was used to estimate hydraulic conductivity reduction with CaCO3 content, considering only the reduction of porosity [103]. By comparing the calculated hydraulic conductivity using the Panda–Lake and Kozeny–Carman models with the measured hydraulic conductivity reported in existing literature, the researchers developed an analytical model that can reasonably predict the hydraulic conductivity of MICP-treated sands for different CaCO3 contents and types of sands [103]. Lin et al. [103] performed an analysis on measured hydraulic conductivity values across various studies identifying that the grain coating (Figure 3b) Panda–Lake model provides reasonable fits to the data provided that the main mechanism is matrix-supporting. The Panda–Lake model takes into account, the shape, tortuosity, specific surface area (surface area of the grain/the volume of the grain), the statistical characteristics of the particle size distribution, the cement saturation of the pore space, the fraction of CaCO3 volume to the total volume of solids, and the specific surface area of the CaCO3 crystals. The matrix-supporting environment seen in Figure 3c shows smaller reduction of hydraulic conductivity with respect to cementation level. It follows, based on the findings of Lin et al. [103] and Konstantinou et al. [76], that the Kozeny–Carman equation would give better estimations of the reduction of hydraulic conductivity for the contact-cementing model. Even though the findings are in good agreement, there is room for further research to examine the combinations of factors and their effects on the resulting hydraulic conductivity. Figure 8 presents the data concentrated from the available references that measured hydraulic conductivity [3,22,51,53,67,76,79,82,83,84,85,86,87,88,89,91,92,93,94,95,96,97,98,99,100,101,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130][3][22][51][53][67][76][79][82][83][84][85][86][87][88][89][91][92][93][94][95][96][97][98][99][100][101][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130]. The y-axis is in a log scale. Despite the fact that there is a trend in reduction similar to the profile presented in Figure 5, the results are scattered showing a weak correlation with the cementation level. This is because not only the volume of cementation is required to identify the reduction in hydraulic conductivity but also the carbonate crystal size and distribution is required. Also, the initial configuration of the granular network is required, and this is the reason the Panda–Lake model performs better compared to the Kozeny–Carman equation. The results also show that UCS has a higher correlation coefficient (0.55) (Figure 5) compared to hydraulic conductivity (with a value of 0.1) showing more dependence on cementation level and less dependence on the granular and cement configuration.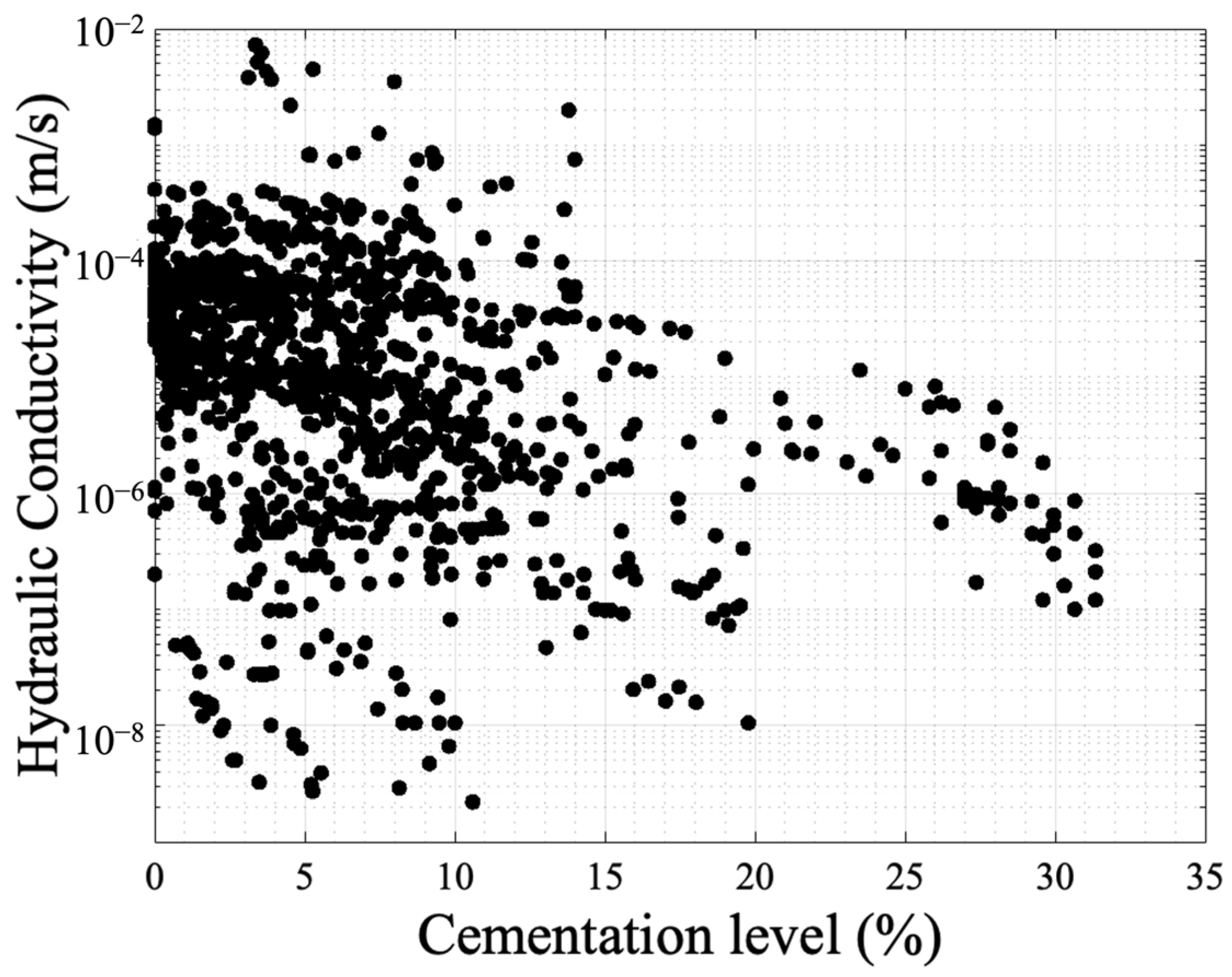 Figure 8.Experimental results of hydraulic conductivity with respect to cementation level as obtained in various studies.
Figure 8.Experimental results of hydraulic conductivity with respect to cementation level as obtained in various studies.
References
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4.
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392.
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423.
- Kawano, J.; Shimobayashi, N.; Kitamura, M.; Shinoda, K.; Aikawa, N. Formation Process of Calcium Carbonate from Highly Supersaturated Solution. J. Cryst. Growth 2002, 237–239, 419–423.
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial Carbonate Precipitation in Construction Materials: A Review. Ecol. Eng. 2010, 36, 118–136.
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281.
- Gollapudi, U.K.; Knutson, C.L.; Bang, S.S.; Islam, M. A New Method for Controlling Leaching through Permeable Channels. Chemosphere 1995, 30, 695–705.
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological Precipitation of CaCO3. Soil. Biol. Biochem. 1999, 31, 1563–1571.
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Dissertation, Murdoch University, Perth, WA, Australia, 2004.
- van Paassen, L. Biogrout: Ground Improvement by Microbially Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009.
- Medici, G.; West, L.J. Reply to Discussion on ‘Review of Groundwater Flow and Contaminant Transport Modelling Approaches for the Sherwood Sandstone Aquifer, UK; Insights from Analogous Successions Worldwide’ by Medici and West (QJEGH, 55, Qjegh2021-176). Q. J. Eng. Geol. Hydrogeol. 2022, 56, qjegh2022-097.
- Burley, S.D. Patterns of Diagenesis in the Sherwood Sandstone Group (Triassic), United Kingdom. Clay Min. 1984, 19, 403–440.
- Bella, G.; Barbero, M.; Barpi, F.; Borri-Brunetto, M.; Peila, D. An Innovative Bio-Engineering Retaining Structure for Supporting Unstable Soil. J. Rock. Mech. Geotech. Eng. 2017, 9, 247–259.
- Konstantinou, C.; Biscontin, G. Soil Enhancement via Microbially Induced Calcite Precipitation. In Proceedings of the 10th International Symposium on Geotechnical Aspects of Underground Construction in Soft Ground, Cambridge, UK, 27–29 June 2022; Taylor & Francis: Cambridge, UK, 2021; pp. 765–772.
- Jiang, N.-J.; Soga, K. The Applicability of Microbially Induced Calcite Precipitation (MICP) for Internal Erosion Control in Gravel–Sand Mixtures. Géotechnique 2017, 67, 42–55.
- Jiang, N.-J.; Soga, K.; Kuo, M. Microbially Induced Carbonate Precipitation for Seepage-Induced Internal Erosion Control in Sand–Clay Mixtures. J. Geotech. Geoenviron. Eng. 2017, 143, 04016100.
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126.
- Erşan, Y.Ç.; Hernandez-Sanabria, E.; Boon, N.; De Belie, N. Enhanced Crack Closure Performance of Microbial Mortar through Nitrate Reduction. Cem. Concr. Compos. 2016, 70, 159–170.
- Hata, T.; Saracho, A.C.; Haigh, S.K.; Yoneda, J.; Yamamoto, K. Microbial-Induced Carbonate Precipitation Applicability with the Methane Hydrate-Bearing Layer Microbe. J. Nat. Gas. Sci. Eng. 2020, 81, 103490.
- Yu, X.; Rong, H. Seawater Based MICP Cements Two/One-Phase Cemented Sand Blocks. Appl. Ocean. Res. 2022, 118, 102972.
- Cui, M.J.; Zheng, J.J.; Chu, J.; Wu, C.C.; Lai, H.J. Bio-Mediated Calcium Carbonate Precipitation and Its Effect on the Shear Behaviour of Calcareous Sand. Acta Geotech. 2021, 16, 1377–1389.
- Lin, W.; Gao, Y.; Lin, W.; Zhuo, Z.; Wu, W.; Cheng, X. Seawater-Based Bio-Cementation of Natural Sea Sand via Microbially Induced Carbonate Precipitation. Environ. Technol. Innov. 2023, 29, 103010.
- Wang, Y.; Konstantinou, C.; Tang, S.; Chen, H. Applications of Microbial-Induced Carbonate Precipitation: A State-of-the-Art Review. Biogeotechnics 2023, 1, 100008.
- Jiang, N.J.; Liu, R.; Du, Y.J.; Bi, Y.Z. Microbial Induced Carbonate Precipitation for Immobilizing Pb Contaminants: Toxic Effects on Bacterial Activity and Immobilization Efficiency. Sci. Total Environ. 2019, 672, 722–731.
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization Based Remediation of As(III) Contaminated Soil by Sporosarcina Ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184.
- Kim, Y.; Kwon, S.; Roh, Y. Effect of Divalent Cations (Cu, Zn, Pb, Cd, and Sr) on Microbially Induced Calcium Carbonate Precipitation and Mineralogical Properties. Front. Microbiol. 2021, 12, 646748.
- He, J.; Chen, X.; Zhang, Q.; Achal, V. More Effective Immobilization of Divalent Lead than Hexavalent Chromium through Carbonate Mineralization by Staphylococcus Epidermidis HJ2. Int. Biodeterior. Biodegrad. 2019, 140, 67–71.
- Li, M.; Cheng, X.; Guo, H. Heavy Metal Removal by Biomineralization of Urease Producing Bacteria Isolated from Soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85.
- Peng, D.; Qiao, S.; Luo, Y.; Ma, H.; Zhang, L.; Hou, S.; Wu, B.; Xu, H. Performance of Microbial Induced Carbonate Precipitation for Immobilizing Cd in Water and Soil. J. Hazard. Mater. 2020, 400, 123116.
- Fang, L.; Niu, Q.; Cheng, L.; Jiang, J.; Yu, Y.Y.; Chu, J.; Achal, V.; You, T. Ca-Mediated Alleviation of Cd2+ Induced Toxicity and Improved Cd2+ Biomineralization by Sporosarcina Pasteurii. Sci. Total Environ. 2021, 787, 147627.
- Rajasekar, A.; Wilkinson, S.; Moy, C.K.S. MICP as a Potential Sustainable Technique to Treat or Entrap Contaminants in the Natural Environment: A Review. Environ. Sci. Ecotechnol. 2021, 6, 100096.
- Torres-Aravena, Á.E.; Duarte-Nass, C.; Azócar, L.; Mella-Herrera, R.; Rivas, M.; Jeison, D. Can Microbially Induced Calcite Precipitation (MICP) through a Ureolytic Pathway Be Successfully Applied for Removing Heavy Metals from Wastewaters? Crystals 2018, 8, 438.
- Qian, X.; Fang, C.; Huang, M.; Achal, V. Characterization of Fungal-Mediated Carbonate Precipitation in the Biomineralization of Chromate and Lead from an Aqueous Solution and Soil. J. Clean. Prod. 2017, 164, 198–208.
- Govarthanan, M.; Mythili, R.; Kamala-Kannan, S.; Selvankumar, T.; Srinivasan, P.; Kim, H. In-Vitro Bio-Mineralization of Arsenic and Lead from Aqueous Solution and Soil by Wood Rot Fungus, Trichoderma Sp. Ecotoxicol. Environ. Saf. 2019, 174, 699–705.
- Zhang, W.; Zhang, H.; Xu, R.; Qin, H.; Liu, H.; Zhao, K. Heavy Metal Bioremediation Using Microbially Induced Carbonate Precipitation: Key Factors and Enhancement Strategies. Front. Microbiol. 2023, 14, 1116970.
- Li, M.; Cheng, H.; Guo, X.; Yang, Z. Biomineralization of Carbonate by Terrabacter Tumescens for Heavy Metal Removal and Biogrouting Applications. J. Environ. Eng. 2015, 142, C4015005.
- Fujita, Y.; Taylor, J.L.; Wendt, L.M.; Reed, D.W.; Smith, R.W. Evaluating the Potential of Native Ureolytic Microbes to Remediate a 90Sr Contaminated Environment. Environ. Sci. Technol. 2010, 44, 7652–7658.
- Lauchnor, E.G.; Schultz, L.N.; Bugni, S.; Mitchell, A.C.; Cunningham, A.B.; Gerlach, R. Bacterially Induced Calcium Carbonate Precipitation and Strontium Coprecipitation in a Porous Media Flow System. Environ. Sci. Technol. 2013, 47, 1557–1564.
- Chen, M.; Li, Y.; Jiang, X.; Zhao, D.; Liu, X.; Zhou, J.; He, Z.; Zheng, C.; Pan, X. Study on Soil Physical Structure after the Bioremediation of Pb Pollution Using Microbial-Induced Carbonate Precipitation Methodology. J. Hazard. Mater. 2021, 411, 125103.
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Bioremediation of Lead-Contaminated Mine Waste by Pararhodobacter Sp. Based on the Microbially Induced Calcium Carbonate Precipitation Technique and Its Effects on Strength of Coarse and Fine Grained Sand. Ecol. Eng. 2017, 109, 57–64.
- Chen, X.; Achal, V. Biostimulation of Carbonate Precipitation Process in Soil for Copper Immobilization. J. Hazard. Mater. 2019, 368, 705–713.
- Kang, C.H.; Kwon, Y.J.; So, J.S. Bioremediation of Heavy Metals by Using Bacterial Mixtures. Ecol. Eng. 2016, 89, 64–69.
- Qiao, S.; Zeng, G.; Wang, X.; Dai, C.; Sheng, M.; Chen, Q.; Xu, F.; Xu, H. Multiple Heavy Metals Immobilization Based on Microbially Induced Carbonate Precipitation by Ureolytic Bacteria and the Precipitation Patterns Exploration. Chemosphere 2021, 274, 129661.
- Mugwar, A.J.; Harbottle, M.J. Toxicity Effects on Metal Sequestration by Microbially-Induced Carbonate Precipitation. J. Hazard. Mater. 2016, 314, 237–248.
- de Oliveira, D.; Horn, E.J.; Randall, D.G. Copper Mine Tailings Valorization Using Microbial Induced Calcium Carbonate Precipitation. J. Environ. Manag. 2021, 298, 113440.
- Konstantinou, C.; Wang, Y.; Biscontin, G.; Soga, K. The Role of Bacterial Urease Activity on the Uniformity of Carbonate Precipitation Profiles of Bio-Treated Coarse Sand Specimens. Sci. Rep. 2021, 11, 6161.
- Zeng, Y.; Chen, Z.; Du, Y.; Lyu, Q.; Yang, Z.; Liu, Y.; Yan, Z. Microbiologically Induced Calcite Precipitation Technology for Mineralizing Lead and Cadmium in Landfill Leachate. J. Environ. Manag. 2021, 296, 113199.
- Han, L.; Li, J.; Xue, Q.; Chen, Z.; Zhou, Y.; Poon, C.S. Bacterial-Induced Mineralization (BIM) for Soil Solidification and Heavy Metal Stabilization: A Critical Review. Sci. Total Environ. 2020, 746, 140967.
- Zhao, C.; Fu, Q.; Song, W.; Zhang, D.; Ahati, J.; Pan, X.; Al-Misned, F.A.; Mortuza, M.G. Calcifying Cyanobacterium (Nostoc Calcicola) Reactor as a Promising Way to Remove Cadmium from Water. Ecol. Eng. 2015, 81, 107–114.
- Gadd, G.M. Bioremedial Potential of Microbial Mechanisms of Metal Mobilization and Immobilization. Curr. Opin. Biotechnol. 2000, 11, 271–279.
- Al Qabany, A.; Soga, K. Effect of Chemical Treatment Used in MICP on Engineering Properties of Cemented Soils. Geotechnique 2013, 63, 331–339.
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2012, 138, 992–1001.
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of Key Environmental Conditions on Microbially Induced Cementation for Soil Stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083.
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-Mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210.
- Mortensen, B.M.; Haber, M.J.; Dejong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of Environmental Factors on Microbial Induced Calcium Carbonate Precipitation. J. Appl. Microbiol. 2011, 111, 338–349.
- Rebata-Landa, V. Microbial Activity in Sediments: Effects on Soil Behavior; Georgia Institute of Technology: Atlanta, GA, USA, 2007.
- Lin, H.; Suleiman, M.T.; Brown, D.G.; Kavazanjian, E. Mechanical Behavior of Sands Treated by Microbially Induced Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2015, 142, 04015066.
- Montoya, B.M.; DeJong, J.T. Stress-Strain Behavior of Sands Cemented by Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2015, 141, 04015019.
- Gao, Y.; Hang, L.; He, J.; Chu, J. Mechanical Behaviour of Biocemented Sands at Various Treatment Levels and Relative Densities. Acta Geotech. 2019, 14, 697–707.
- Feng, K.; Montoya, B.M. Influence of Confinement and Cementation Level on the Behavior of Microbial-Induced Calcite Precipitated Sands under Monotonic Drained Loading. J. Geotech. Geoenviron. Eng. 2015, 142, 04015057.
- Cui, M.J.; Zheng, J.J.; Zhang, R.J.; Lai, H.J.; Zhang, J. Influence of Cementation Level on the Strength Behaviour of Bio-Cemented Sand. Acta Geotech. 2017, 12, 971–986.
- Konstantinou, C.; Biscontin, G.; Logothetis, F. Tensile Strength of Artificially Cemented Sandstone Generated via Microbially Induced Carbonate Precipitation. Materials 2021, 14, 4735.
- Xiao, Y.; He, X.; Evans, T.M.; Stuedlein, A.W.; Liu, H. Unconfined Compressive and Splitting Tensile Strength of Basalt Fiber-Reinforced Biocemented Sand. J. Geotech. Geoenviron. Eng. 2019, 145, 04019048.
- Pakbaz, M.S.; Kolahi, A.; Ghezelbash, G. Assessment of Microbial Induced Calcite Precipitation (MICP) in Fine Sand Using Native Microbes under Both Aerobic and Anaerobic Conditions. KSCE J. Civ. Eng. 2022, 26, 1051–1065.
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2017, 34, 524–537.
- Pan, X.; Chu, J.; Yang, Y.; Cheng, L. A New Biogrouting Method for Fine to Coarse Sand. Acta Geotech. 2019, 15, 1–16.
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-Cementation of Sandy Soil Using Microbially Induced Carbonate Precipitation for Marine Environments. Geotechnique 2014, 64, 1010–1013.
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. A Microfluidic Chip and Its Use in Characterising the Particle-Scale Behaviour of Microbial-Induced Calcium Carbonate Precipitation (MICP). Geotechnique 2019, 69, 1086–1094.
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. Microscale Visualization of Microbial-Induced Calcium Carbonate Precipitation Processes. J. Geotech. Geoenviron. Eng. 2019, 145, 04019045.
- Wang, Y. Microbial-Induced Calcium Carbonate Precipitation: From Micro to Macro Scale. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2018.
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. Effects of Bacterial Density on Growth Rate and Characteristics of Microbial-Induced CaCO3 Precipitates: A Particle-Scale Experimental Study. ASCE J. Geotech. Geoenviron. Eng. 2021, 147, 04021036.
- Wang, Y.; Konstantinou, C.; Soga, K.; Biscontin, G.; Kabla, A.J. Use of Microfluidic Experiments to Optimize MICP Treatment Protocols for Effective Strength Enhancement of MICP-Treated Sandy Soils. Acta Geotech. 2022, 17, 3817–3838.
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale Investigations of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP) in the Temperature Range 4–50 °C. Acta Geotech. 2023, 18, 2239–2261.
- Wang, Y.; Wang, Y.; Konstantinou, C. Strength Behavior of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP)-Treated Soil. J. Geotech. Geoenviron. Eng. 2023.
- Wang, Y.; Wang, Y.; Konstantinou, C. Effects of Environmental Temperature on the Effectiveness of Microbially Induced Carbonate Precipitation. ESS Open Archive. 2022.
- Konstantinou, C.; Wang, Y.; Biscontin, G. A Systematic Study on the Influence of Grain Characteristics on Hydraulic and Mechanical Performance of MICP-Treated Porous Media. Transp. Porous Media 2023, 147, 305–330.
- Terzis, D.; Laloui, L. 3-D Micro-Architecture and Mechanical Response of Soil Cemented via Microbial-Induced Calcite Precipitation. Sci. Rep. 2018, 8, 1416.
- Xiao, Y.; Asce, M.; Stuedlein, A.W.; Asce, M.; Ran, J.; Evans, T.M.; Asce, A.M.; Cheng, L.; Liu, H.; Paassen, L.A.V.; et al. Effect of Particle Shape on Strength and Stiffness of Biocemented Glass Beads. J. Geotech. Geoenviron. Eng. 2019, 145, 06019016.
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of Sand Soil by Microbially Induced Calcite Precipitation at Various Degrees of Saturation. Can. Geotech. J. 2013, 50, 81–90.
- Dawoud, O. The Applicability of Microbially Induced Calcite Precipitation (MICP) for Soil Treatment. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2015.
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728.
- Konstantinou, C. Hydraulic Fracturing of Artificially Generated Soft Sandstones. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2021.
- Yasuhara, H.; Hayashi, K.; Okamura, M. Evolution in mechanical and hydraulic properties of calcite-cemented sand mediated by biocatalyst. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 3984–3992.
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental Optimization of Microbial-Induced Carbonate Precipitation for Soil Improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598.
- Zamani, A.; Montoya, B.; Gabr, M.A. Investigating the Challenges of in Situ Delivery of MICP in Fine Grain Sands and Silty Sand. Can. Geotech. J. 2019, 56, 1889–1900.
- Li, Y.; Chen, J. Experimental Study on the Permeability of Microbial-Solidified Calcareous Sand Based on MICP. Appl. Sci. 2022, 12, 11447.
- Choi, S.G.; Hoang, T.; Park, S.S. Undrained Behavior of Microbially Induced Calcite Precipitated Sand with Polyvinyl Alcohol Fiber. Appl. Sci. 2019, 9, 1214.
- Choi, S.-G.; Wu, S.; Chu, J. Biocementation for Sand Using an Eggshell as Calcium Source. J. Geotech. Geoenviron. Eng. 2016, 142, 06016010.
- Gomez, M.G.; Anderson, C.M.; Dejong, J.T.; Nelson, D.C.; Lau, X.H. Stimulating In-Situ Soil Bacteria for Bio-Cementation of Sands. In Proceedings of the Geo-Congress, Atlanta, GA, USA, 23–26 February 2014; pp. 1674–1682.
- Dawoud, O. Modification of Hydraulic Conductivity of Sandy Soil Using Seawater and Alkaline Solutions. IOP Conf. Ser. Mater. Sci. Eng. 2020, 800, 012011.
- Dawoud, O.; Chen, C.Y.; Soga, K. Microbial induced calcite precipitation for geotechnical and environmental applications. In New Frontiers in Geotechnical Engineering; American Society of Civil Engineers: Reston, VA, USA, 2014; pp. 11–18.
- Song, C.; Song, C.; Chen, Y.; Wang, J. Plugging High-Permeability Zones of Oil Reservoirs by Microbially Mediated Calcium Carbonate Precipitation. ACS Omega 2020, 5, 14376–14383.
- Choi, S.G.; Chang, I.; Lee, M.; Lee, J.H.; Han, J.T.; Kwon, T.H. Review on Geotechnical Engineering Properties of Sands Treated by Microbially Induced Calcium Carbonate Precipitation (MICP) and Biopolymers. Constr. Build. Mater. 2020, 246, 118415.
- Eryürük, K. Effect of Cell Density on Decrease in Hydraulic Conductivity by Microbial Calcite Precipitation. AMB Express 2022, 12, 104.
- Duo, L.; Kan-liang, T.; Hui-li, Z.; Yu-yao, W.; Kang-yi, N.; Shi-can, Z. Experimental Investigation of Solidifying Desert Aeolian Sand Using Microbially Induced Calcite Precipitation. Constr. Build. Mater. 2018, 172, 251–262.
- Kadhim, F.J.; Zheng, J.-J. Influences of Calcium Sources and Type of Sand on Microbial Induced Carbonate Precipitation. Int. J. Adv. Eng. Technol. 2017, 10, 20–29.
- Akoğuz, H.; Çelik, S.; Bariş, Ö. The Effects of Different Sources of Calcium in Improvement of Soils by Microbially Induced Calcite Precipitation (MICP). Sigma J. Eng. Nat. Sci. 2019, 37, 953–965.
- Dekuyer, A.; Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Calcium Carbonate Induced Precipitation for Soil Improvement by Urea Hydrolysing Bacteria. In Proceedings of the Advances in Civil, Environmental, and Materials Research (ACEM’ 12), Seoul, South Korea, 26–29 August 2012.
- Rowshanbakht, K.; Khamehchiyan, M.; Sajedi, R.H.; Nikudel, M.R. Effect of Injected Bacterial Suspension Volume and Relative Density on Carbonate Precipitation Resulting from Microbial Treatment. Ecol. Eng. 2016, 89, 49–55.
- Tian, K.; Wu, Y.; Zhang, H.; Li, D.; Nie, K.; Zhang, S. Increasing Wind Erosion Resistance of Aeolian Sandy Soil by Microbially Induced Calcium Carbonate Precipitation. Land Degrad. Dev. 2018, 29, 4271–4281.
- Song, C.; Elsworth, D.; Zhi, S.; Wang, C. The Influence of Particle Morphology on Microbially Induced CaCO3 Clogging in Granular Media. Mar. Georesources Geotechnol. 2021, 39, 74–81.
- Panda, M.N.; Lake, L.W. A Physical Model of Cementation and Its Effects on Single-Phase Permeability. Am. Assoc. Pet. Geol. Bull. 1995, 79, 431–443.
- Lin, H.; Suleiman, M.T.; Brown, D.G. Investigation of Pore-Scale CaCO3 Distributions and Their Effects on Stiffness and Permeability of Sands Treated by Microbially Induced Carbonate Precipitation (MICP). Soils Found. 2020, 60, 944–961.
- Song, C.; Elsworth, D.; Jia, Y.; Lin, J. Permeable Rock Matrix Sealed with Microbially-Induced Calcium Carbonate Precipitation: Evolutions of Mechanical Behaviors and Associated Microstructure. Eng. Geol. 2022, 304, 106697.
- Wang, X.; Nackenhorst, U. A Coupled Bio-Chemo-Hydraulic Model to Predict Porosity and Permeability Reduction during Microbially Induced Calcite Precipitation. Adv. Water Resour. 2020, 139, 103563.
- Montoya, B.M.; Safavizadeh, S.; Gabr, M.A. Enhancement of Coal Ash Compressibility Parameters Using Microbial-Induced Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2019, 145, 04019018.
- Phang, I.R.K.; Wong, K.S.; Chan, Y.S.; Lau, S.Y. Effect of Microbial-Induced Calcite Precipitation towards Strength and Permeability of Peat. Bull. Eng. Geol. Environ. 2022, 81, 314.
- Zhao, Y.; Xiao, Z.; Fan, C.; Shen, W.; Wang, Q.; Liu, P. Comparative Mechanical Behaviors of Four Fiber-Reinforced Sand Cemented by Microbially Induced Carbonate Precipitation. Bull. Eng. Geol. Environ. 2020, 79, 3075–3086.
- Tian, K.; Wang, X.; Zhang, S.; Zhang, H.; Zhang, F.; Yang, A. Effect of Reactant Injection Rate on Solidifying Aeolian Sand via Microbially Induced Calcite Precipitation. J. Mater. Civ. Eng. 2020, 32, 04020291.
- Yu, X.; He, Z.; Li, X. Bio-Cement-Modified Construction Materials and Their Performances. Environ. Sci. Pollut. Res. 2022, 29, 11219–11231.
- Sharma, M.; Satyam, N.; Reddy, K.R. Hybrid Bacteria Mediated Cemented Sand: Microcharacterization, Permeability, Strength, Shear Wave Velocity, Stress-Strain, and Durability. Int. J. Damage Mech. 2021, 30, 618–645.
- Yang, B.; Li, H.; Li, H.; Ge, N.; Ma, G.; Zhang, H.; Zhang, X.; Zhuang, L. Experimental Investigation on the Mechanical and Hydraulic Properties of Urease Stabilized Fine Sand for Fully Permeable Pavement. Int. J. Transp. Sci. Technol. 2022, 11, 60–71.
- Yang, Y.; Chu, J.; Xiao, Y.; Liu, H.; Cheng, L. Seepage Control in Sand Using Bioslurry. Constr. Build. Mater. 2019, 212, 342–349.
- Fang, X.; Yang, Y.; Chen, Z.; Liu, H.; Xiao, Y.; Shen, C. Influence of Fiber Content and Length on Engineering Properties of MICP-Treated Coral Sand. Geomicrobiol. J. 2020, 37, 582–594.
- Choi, S.G.; Chu, J.; Brown, R.C.; Wang, K.; Wen, Z. Sustainable Biocement Production via Microbially Induced Calcium Carbonate Precipitation: Use of Limestone and Acetic Acid Derived from Pyrolysis of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5183–5190.
- Ma, G.; He, X.; Jiang, X.; Liu, H.; Chu, J.; Xiao, Y. Strength and Permeability of Bentonite-Assisted Biocemented Coarse Sand. Can. Geotech. J. 2021, 58, 969–981.
- Wen, K.; Li, Y.; Liu, S.; Bu, C.; Li, L. Development of an Improved Immersing Method to Enhance Microbial Induced Calcite Precipitation Treated Sandy Soil through Multiple Treatments in Low Cementation Media Concentration. Geotech. Geol. Eng. 2019, 37, 1015–1027.
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 04014006.
- Safavizadeh, S.; Montoya, B.M.; Gabr, M.A. Effect of Microbial Induced Calcium Carbonate Precipitation on Compressibility and Hydraulic Conductivity of Fly Ash. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 69–79.
- Rajasekar, A.; Moy, C.K.S.; Wilkinson, S.; Sekar, R. Microbially Induced Calcite Precipitation Performance of Multiple Landfill Indigenous Bacteria Compared to a Commercially Available Bacteria in Porous Media. PLoS ONE 2021, 16, e0254676.
- Zhao, Y.; Zhang, P.; Fang, H.; Guo, C.; Zhang, B.; Wang, F. Bentonite-Assisted Microbial-Induced Carbonate Precipitation for Coarse Soil Improvement. Bull. Eng. Geol. Environ. 2021, 80, 5623–5632.
- Jawad, F.; Zheng, J.-J. Improving Poorly Graded Fine Sand with Microbial Induced Calcite Precipitation. Br. J. Appl. Sci. Technol. 2016, 17, 1–9.
- Montoya, B.M.; Do, J.; Gabr, M.M. Erodibility of Microbial Induced Carbonate Precipitation-Stabilized Sand under Submerged Impinging Jet. In Proceedings of the IFCEE, Orlando, FL, USA, 5–10 March 2018; pp. 19–28.
- Niu, J.G.; Liang, S.H.; Gong, X.; Feng, D.L.; Luo, Q.Z.; Dai, J. Experimental Study on the Effect of Grouting Interval on Microbial Induced Calcium Carbonate Precipitation. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012071.
- Gong, X.; Niu, J.; Liang, S.; Feng, D.; Luo, Q. Environmental Effect of Grouting Batches on Microbial-Induced Calcite Precipitation. Ekoloji Derg. 2019, 28, 929–936.
- Choi, S.G.; Wang, K.; Chu, J. Properties of Biocemented, Fiber Reinforced Sand. Constr. Build. Mater. 2016, 120, 623–629.
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and Predictions of Physical Properties of Sand Cemented by Enzymatically-Induced Carbonate Precipitation. Soils Found. 2012, 52, 539–549.
- Sidik, W.S.; Canakci, H.; Kilic, I.H.; Celik, F. Applicability of Biocementation for Organic Soil and Its Effect on Permeability. Geomech. Eng. 2014, 7, 649–663.
- Zamani, A.; Asce, S.M.; Montoya, B.M.; Asce, M. Shearing and Hydraulic Behavior of MICP Treated Silty Sand. In Proceedings of the Geotechnical Frontiers 2017, Orlando, FL, USA, 12–15 March 2017; pp. 290–299.
- Stabnikov, V.; Jian, C.; Ivanov, V.; Li, Y. Halotolerant, Alkaliphilic Urease-Producing Bacteria from Different Climate Zones and Their Application for Biocementation of Sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460.
More
