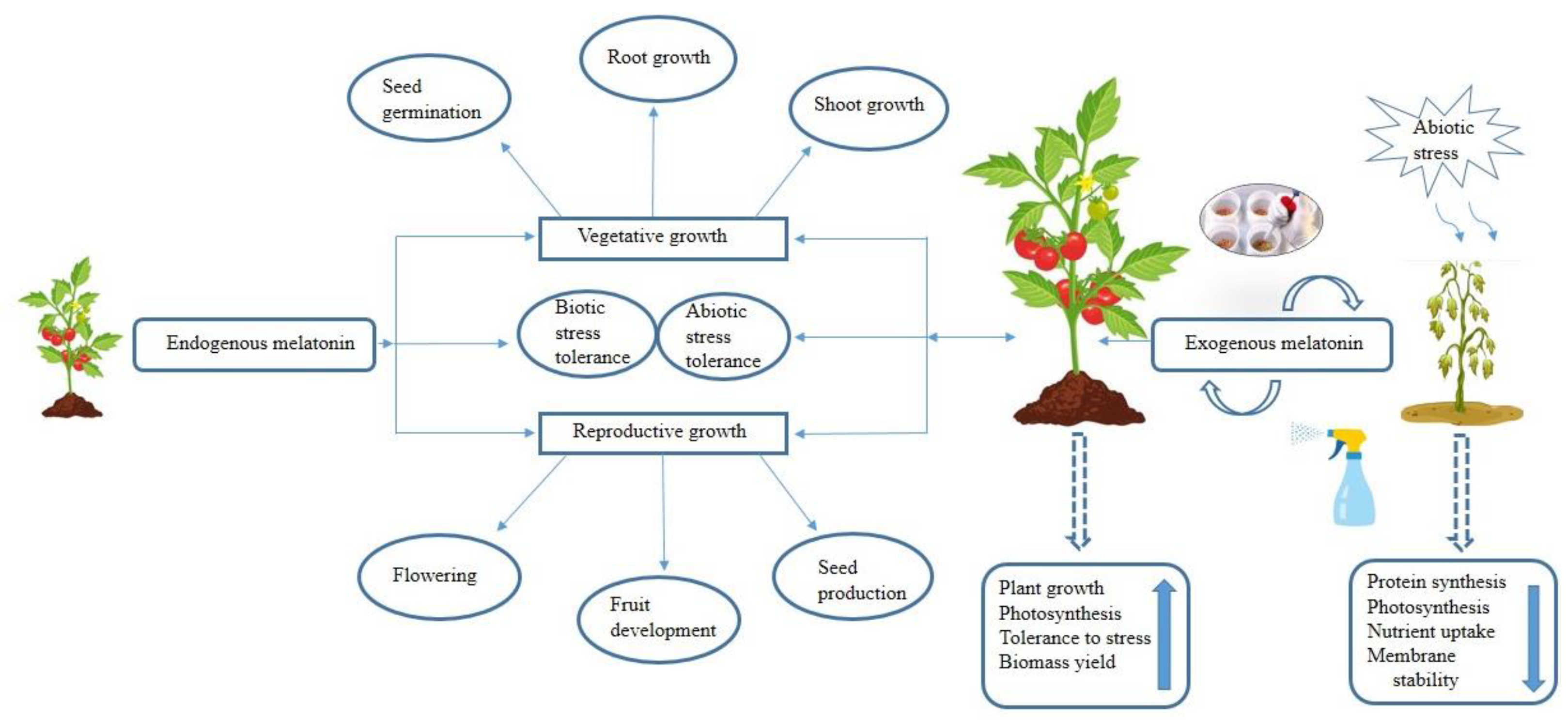
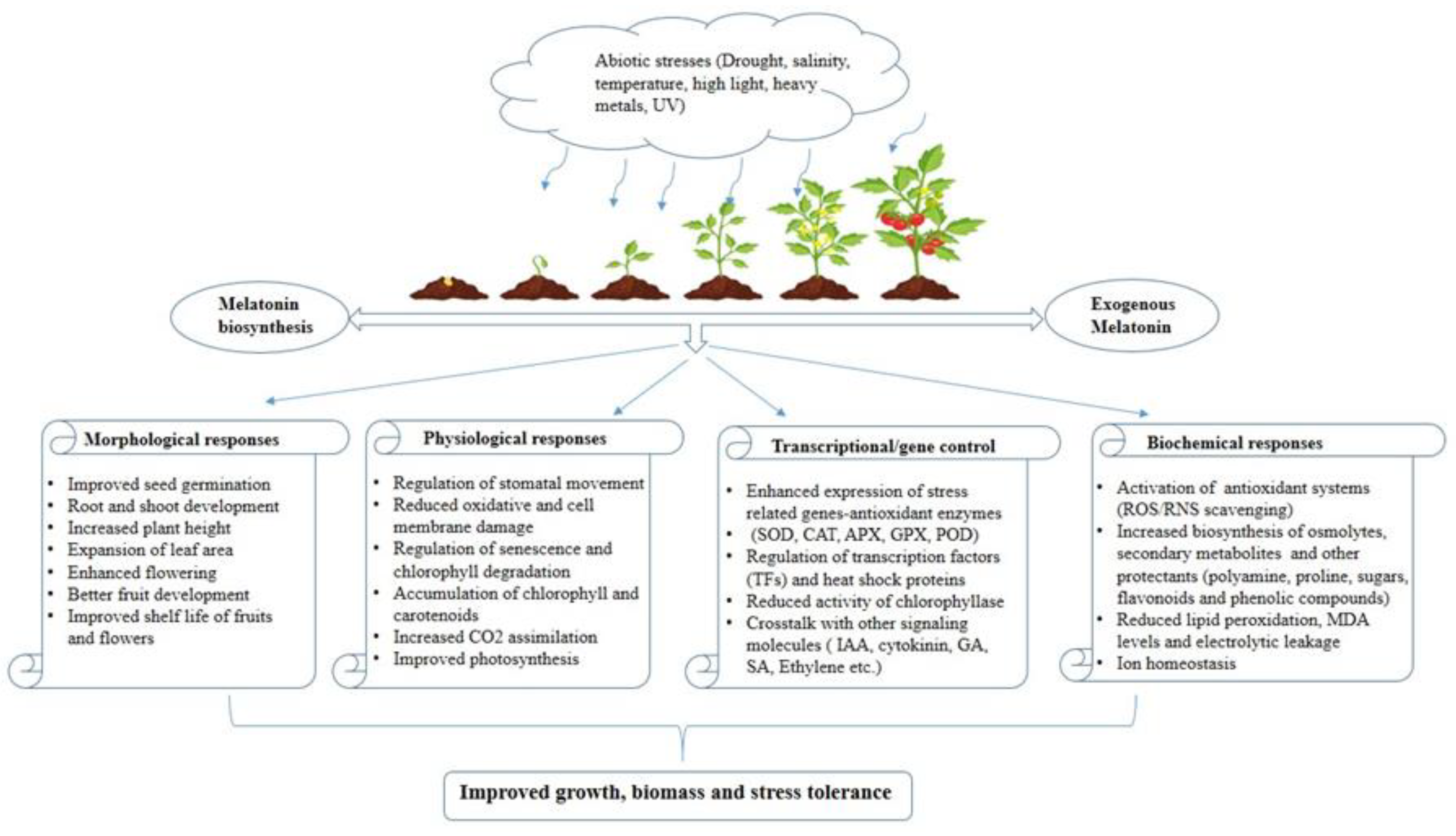
Extensive research has shed light on the dual role of melatonin in plants, where it serves as both a growth regulator, fostering growth and development, and a potent protector against abiotic stresses. The inherent potential of melatonin to function as a natural antioxidant positions it as a promising biostimulant for agricultural use, bolstering plants’ abilities to withstand a wide array of environmental challenges. Beyond its antioxidant properties, melatonin has demonstrated its capacity to regulate the expression of genes associated with the photosynthetic process. This additional characteristic enhances its appeal as a versatile chemical agent that can be exogenously applied to plants, particularly in adverse conditions, to improve their resilience and optimize photosynthetic efficiency in every phase of the plant life cycle.
- melatonin
- exogenous
- abiotic stress
- photosynthesis
- antioxidant
1. Introduction
2. Drought
3. Salinity
4. Temperature
5. High Light Intensity
6. Metal Toxicity
7. UV Radiation
References
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570.
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127.
- Arnao, M.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150.
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875.
- Ren, S.; Jiang, G.L.; Rutto, L. Melatonin priming enhances symbiotic nitrogen fixation in soybean, Glycine max L. J. Biotech Res. 2019, 10, 136–144.
- Yang, S.; Zhao, Y.; Qin, X.; Ding, C.; Chen, Y.; Tang, Z.; Huang, Y.; Reiter, R.J.; Yuan, S.; Yuan, M. New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 2022, 73, 5918–5927.
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101.
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528.
- Li, C.; Tan, D.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680.
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167.
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100.
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437.
- Yang, X.L.; Xu, H.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2018, 56, 884–892.
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45.
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368.
- Imran, M.; Mpovo, C.L.; Aaqil Khan, M.; Shaffique, S.; Ninson, D.; Bilal, S.; Khan, M.; Kwon, E.H.; Kang, S.M.; Yun, B.W.; et al. Synergistic Effect of Melatonin and Lysinibacillus fusiformis L. (PLT16) to Mitigate Drought Stress via Regulation of Hormonal, Antioxidants System, and Physio-Molecular Responses in Soybean Plants. Int. J. Mol. Sci. 2023, 24, 8489.
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47.
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43.
- Karaca, P.; Cekic, F.Ö. Exogenous melatonin-stimulated defense responses in tomato plants treated with polyethylene glycol. Int. J. Veg. Sci. 2019, 25, 601–609.
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149.
- Cherono, S.; Ntini, C.; Wassie, M.; Dulal Mollah, M.; Belal, M.A.; Ogutu, C.; Han, Y. Exogenous application of melatonin improves drought tolerance in coffee by regulating photosynthetic efficiency and oxidative damage. J. Am. Soc. Hortic. Sci. 2021, 146, 24–32.
- Alyammahi, O.; Gururani, M.A. Chlorophyll-a fluorescence analysis reveals differential response of photosynthetic machinery in melatonin-treated oat plants exposed to osmotic stress. Agronomy 2020, 10, 1520.
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem ii in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677.
- Li, D.; Zhang, D.; Wang, H.; Li, Y.; Li, R. Physiological response of plants to polyethylene glycol (PEG-6000) by exogenous melatonin application in wheat. Zemdirbyste 2017, 104, 219–228.
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597.
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026.
- Luo, Y.; Hu, T.; Huo, Y.; Wang, L.; Zhang, L.; Yan, R. Effects of Exogenous Melatonin on Chrysanthemum Physiological Characteristics and Photosynthesis under Drought Stress. Horticulturae 2023, 9, 106.
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18.
- Eisa, E.A.; Honfi, P.; Tilly-Mándy, A.; Gururani, M.A. Exogenous Application of Melatonin Alleviates Drought Stress in Ranunculus asiaticus by Improving Its Morphophysiological and Biochemical Attributes. Horticulturae 2023, 9, 262.
- Li, G.; Li, Y.; Zhu, Y.; Zheng, W.; Li, M.; Hu, J.; Fei, Y.; Zhu, S. Exogenous application of melatonin to mitigate drought stress-induced oxidative damage in Phoebe sheareri seedlings. PeerJ 2023, 11, e15159.
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27.
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J. Plant Interact. 2019, 14, 453–463.
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M. Melatonin and Gibberellic Acid Promote Growth and Chlorophyll Biosynthesis by Regulating Antioxidant and Methylglyoxal Detoxification System in Tomato Seedlings Under Salinity. J. Plant Growth Regul. 2020, 39, 1488–1502.
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant Sci. 2016, 7, 1823.
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886.
- ElSayed, A.; Rafudeen, M.; Gomaa, A.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381.
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375.
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331.
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 2018, 9, 914.
- Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings. Antioxidants 2022, 11, 758.
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of avena sativa during salinity stress. Plants 2019, 8, 610.
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front. Plant Sci. 2018, 9, 998.
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedlings under Cold Stress. Antioxidants 2022, 11, 2414.
- Meng, J.F.; Yu, Y.; Shi, T.C.; Fu, Y.S.; Zhao, T.; Zhang, Z.W. Melatonin treatment of pre-veraison grape berries modifies phenolic components and antioxidant activity of grapes and wine. Food Sci. Technol. 2019, 39, 35–42.
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809.
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’ippolito, I.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778.
- Fan, X.; Zhao, J.; Sun, X.; Zhu, Y.; Li, Q.; Zhang, L.; Zhao, D.; Huang, L.; Zhang, C.; Liu, Q. Exogenous Melatonin Improves the Quality Performance of Rice under High Temperature during Grain Filling. Agronomy 2022, 12, 949.
- Xu, W.; Cai, S.Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Reiter, R.J.; et al. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469.
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin Pretreatment Confers Heat Tolerance and Repression of Heat-Induced Senescence in Tomato Through the Modulation of ABA- and GA-Mediated Pathways. Front. Plant Sci. 2021, 12, 650955.
- Sun, C.; Meng, S.; Wang, B.; Zhao, S.; Liu, Y.; Qi, M.; Wang, Z.; Yin, Z.; Li, T. Exogenous melatonin enhances tomato heat resistance by regulating photosynthetic electron flux and maintaining ROS homeostasis. Plant Physiol. Biochem. 2023, 196, 197–209.
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 2017, 61, 571–578.
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785.
- Zhou, R.; Wan, H.; Jiang, F.; Li, X.; Yu, X.; Rosenqvist, E.; Ottosen, C.O. The alleviation of photosynthetic damage in tomato under drought and cold stress by high CO2 and melatonin. Int. J. Mol. Sci. 2020, 21, 5587.
- Chang, J.; Guo, Y.; Zhang, Z.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; Li, H. CBF-responsive pathway and phytohormones are involved in melatonin-improved photosynthesis and redox homeostasis under aerial cold stress in watermelon. Acta Physiol. Plant. 2020, 42, 159.
- Yang, S.J.; Huang, B.; Zhao, Y.Q.; Hu, D.; Chen, T.; Ding, C.B.; Chen, Y.E.; Yuan, S.; Yuan, M. Melatonin Enhanced the Tolerance of Arabidopsis thaliana to High Light Through Improving Anti-oxidative System and Photosynthesis. Front. Plant Sci. 2021, 12, 752584.
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci. Rep. 2015, 5, 10919.
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601.
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous Melatonin Improves Physiological Characteristics and Promotes Growth of Strawberry Seedlings Under Cadmium Stress. Hortic. Plant J. 2021, 7, 13–22.
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-zno stress. Molecules 2017, 22, 1727.
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593.
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Noor, Y.; Kanwal, A.; Khalid, A.; Javed, M.; et al. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364.
- Yang, X.; Ren, J.; Lin, X.; Yang, Z.; Deng, X.; Ke, Q. Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 3816.
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641.
- Haskirli, H.; Yilmaz, O.; Ozgur, R.; Uzilday, B.; Turkan, I. Melatonin mitigates UV-B stress via regulating oxidative stress response, cellular redox and alternative electron sinks in Arabidopsis thaliana. Phytochemistry 2021, 182, 112592.
- Zhao, Z.; Yun, C.; Gu, L.; Liu, J.; Yao, L.; Wang, W.; Wang, H. Melatonin enhances biomass, phenolic accumulation, and bioactivities of rosemary (Rosmarinus officinalis) in vitro shoots under UV-B stress. Physiol. Plant. 2023, 175, e13956.
