Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by László Vécsei and Version 3 by Camila Xu.
Multiple sclerosis (MS) is a chronic disease characterised by inflammation, extensive primary demyelination and progressive neurodegenerative processes.
- glutamate excitotoxicity
- lymphocyte
- microglia
- mitochondrial dysfunction
- multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is a chronic disease characterised by inflammation, extensive primary demyelination and progressive neurodegenerative processes [1]. Although a wealth of interesting information on the pathogenesis of MS has been accumulated over the past decades, the exact cause of this disease remains to be elucidated. In addition to environmental and genetic factors, much interest has focused on the role of various infectious agents. In particular, the role of the herpes virus family, Epstein-Barr virus (EBV) and human herpesvirus 6 (HHV-6A) has been the subject of much research interest. A recently published study suggests that age-dependent EBV infection may contribute to the development of MS, while based on the presence of HHV-6A antibodies suggests that the development of MS is independent of age [2].
In everyday medical practice, MS has two main clinical presentations: relapsing-remitting MS (RRMS) and progressive form (PMS). The first clinical presentation of MS is a clinically isolated syndrome (CIS), which falls within the spectrum of RRMS. The progressive form includes primary progressive multiple sclerosis (PPMS) and secondary progressive multiple sclerosis (SPMS) [3]. A better understanding of the pathomechanisms of RRMS and SPMS and, just as important, a better understanding of the molecular mechanism of the transition from RRMS to SPMS are some of the major challenges in MS research. Recently published results have confirmed that long-term disability in MS is largely independent of relapses (progression independent of relapse activity, PIRA) and correlates well with brain atrophy detected on MRI images. Particularly relevant in this regard is the study by Cree et al., which included 138 patients. In this group, 92 patients diagnosed with RRMS had evidence of silent progression, which could certainly be related to the neurodegenerative process in RRMS [4]. This silent progression is likely to be present in many patients diagnosed with RRMS. The same factors are likely to be responsible for the clinical symptoms of SPMS when clinical worsening is clearly evident. Therefore, the pathophysiological events in SPMS likely occur much earlier than is currently thought. A better understanding of this process also has important implications for therapeutic decision-making.
Significant progress has been made in recent years in understanding the pathomechanism of MS. However, little is known about the underlying cellular and molecular mechanisms that influence lesion formation and progression in individual patients. Understanding the role of these pathobiochemical markers would be important, as they may, in many cases, have a major impact on correct therapeutic decisions and gain a better insight into the relationship between the clinical classification and the pathomechanism of MS.
2. MS Lesion Pathology
The pathological hallmarks of MS lesions are inflammation, demyelination, axonal damage and gliosis. The pathological process involves the formation of lesions in the central nervous system (CNS) consisting of lymphocytes, macrophages and glial cells, leading to demyelination and axonal loss. MS plaques form in the brain and spinal cord, mainly, but not exclusively, in the white matter around the ventricles, the optic nerves and tracts, the corpus callosum, the cerebellar peduncles, the long tracts, and the spinal cord and brainstem. They are present in all forms of MS, although their quantities and composition vary between the different forms of MS and as the disease progresses. Different types of lesions (see Figure 1) can be distinguished at different stages of MS based on their degree of microglial activation, inflammatory and demyelinating activity [5]. According to neuropathological data, pre-active lesions are characterised by clusters of activated microglia in areas of otherwise normal-appearing myelin [6]. Active MS lesions show infiltration of lymphocytes around a central vein, accompanied by microglial activation and the presence of macrophages attracted to the lesion, which produce pro-inflammatory cytokines and inflammatory mediators. Therefore, are thought to be involved in the development of chronic axonal loss. B-cells and plasma cells remain mainly in the perivascular space, while CD8+ T-cells diffusely infiltrate the lesion parenchyma [7][8][7,8]. Macrophages and microglia contain myelin degradation products, indicating that they facilitate the degradation of myelin proteins [9][10][9,10]. These lesions are most common in early MS (acute and RRMS) but become rare during progressive MS [11], and there is marked inflammation, significant damage to the blood–brain barrier (BBB) and concomitant diffuse active demyelination in the lesion associated with massive microglial infiltration [1]. However, with the impenetrable BBB in the CNS, compartmentalised inflammation may persist as chronic inflammation. Most acute inflammatory lesions in MS subsequently become inactive (chronic inactive lesions) and shrink due to gliosis. This chronic inflammation is thought to underlie the development of so-called chronic active lesions (also commonly referred to as “mixed active–inactive” or “smouldering” lesions) [7][11][12][7,11,12]. Smouldering lesions show a low-grade chronic inflammation characterised by chronic axonal damage and concurrent demyelination and are further characterised by a gradual increase in size towards the normal-appearing white matter (NAWM) [11][13][11,13]. Based on autopsies, 20–40% of white matter lesions are such lesions, with CD8+ T- and CD20+ B lymphocytes in the centre with a small number of plasma cells, surrounded by a loose network of iron-loaded activated microglia cells and macrophages and proliferating oligodendrocyte cells at the periphery [8][14][8,14].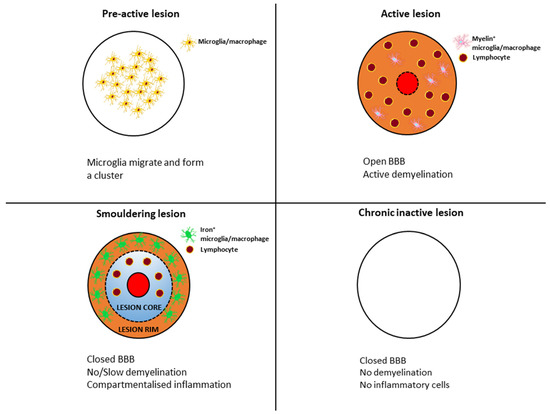
Figure 1.
Transformation of typical lesion patterns in the course of multiple sclerosis.
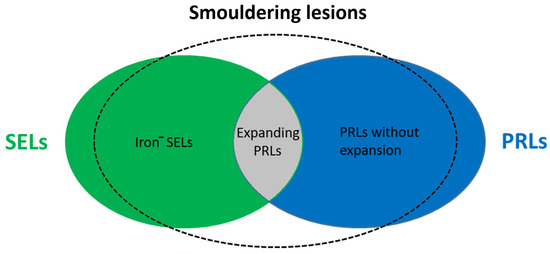
Figure 2.
Representation of smouldering lesions in MS. Iron
−
—iron negative; SEL—slowly expanding lesion; PRL—paramagnetic rim lesion.
