Because of the immunoregulatory properties of cannabinoids, the endocannabinoid system (ECS) may have an important role in shaping the tumor microenvironment (TME). Members of the ECS, an entity that consists of cannabinoid receptors, endocannabinoids and their synthesizing/degrading enzymes, have been associated with both tumor growth and rejection. Immune cells express cannabinoid receptors and produce endocannabinoids, thereby forming an “immune endocannabinoid system”. Although in vitro effects of exogenous cannabinoids on immune cells are well described, the role of the ECS in the TME, and hence in tumor development and immunotherapy, is still elusive.
- Immune Endocannabinoid System
- tumor microenvironment
- cancer
1. Introduction
Gene mutations either caused by inheritance, environmental influence, faulty DNA replication or epigenetic modifications, and the accumulation and aberrant activity of these genes are key features in the process of cancer development [1][2]. Cells that aberrantly express these genes are constantly recognized and subsequently eradicated by cells of the immune system during tumorigenesis in a process called immune surveillance [3]. Nonetheless, mutated cells escape this process and succeed in developing cancer through the selection of tumor cell variants that either lack immunogenic features of recognition or exhibit features for the suppression of the evoked immune response [4].
Maintenance of tissue homeostasis is the work of immune cells, fibroblasts, the vasculature and extracellular matrix components. Apart from cancer cells, neoplastic lesions contain additional cell types, such as endothelial cells, pericytes, cancer-associated fibroblasts and immune cells [5]. Together, they can serve as a hurdle of cancer development [6]. Similar to inflammation, aberrant signaling, driven by cytokines and lipid mediators, among them also endocannabinoids, cause changes in tissue homeostasis and a shift towards a pro-tumorigenic environment and eventually to the development of cancer [6][7]. Thus, ongoing inflammation constitutes one of the hallmarks of cancer [5]. Like in inflammation, cells of the innate and adaptive immunity infiltrate tumors to form the immune tumor microenvironment (TME) with the aim to combat neoplastic growth [8]. Many of these cells express components of the endocannabinoid system (ECS), such as cannabinoid receptors [9][10][11][12]. Immune cells interact with each other and with tumor cells, they react to other components of the TME and the ECS, and they can subsequently halt but also contribute to tumor progression in experimental and clinical cancer [8][13]. All types of immune cells can be observed in tumors, including macrophages, dendritic cells (DCs), neutrophils, eosinophils, mast cells, natural killer (NK) cells, and B and T cells (including Th cells, and cytotoxic T cells) [8]. Importantly, disease-free and overall survival critically depends on the immune cell compositions within the TME [8].
2. The Endocannabinoid System and the Tumor Microenvironment
Receptors and enzymes of the ECS have been mostly measured and quantified by immunohistochemical, Western blot and PCR methods using tissue from a variety of tumor models and biopsies from patients with, e.g., breast, brain, prostate, colon and cervical cancer. Each of the tumors may exhibit either up- or down-regulation of cannabinoid receptors (which are often increased in tumors), and of endocannabinoids and their metabolizing enzymes, FAAH and MGL (rev. in [14]). Correlations between expression of cannabinoid receptors and disease outcome largely differ between various types of cancer [14] indicating that there is no universal (e.g., anti-carcinogenic) role of the ECS in tumor development but that its role rather depends on the type of the tumor. For instance, CB2 overexpression in HER-2 positive breast cancer is a marker for poor outcome [15], whereas in hepatocarcinoma, CB1 and CB2 expression correlate with good clinical outcome [16].
(Endo)cannabinoids have direct anti-carcinogenic effects on tumor cells [17][18][19]. These effects include inhibition of proliferation, cell cycle arrest, apoptosis and autophagy [19][20]. Thus, AEA- and 2-AG-dependent anti-proliferative effects have been demonstrated in colon, breast, prostate and cervical cancer cells [14][21][22]. Many of these studies were also conducted with exogenous cannabinoids such as Δ9-THC, which mimics the effects of endocannabinoids on cannabinoid receptors [17]. In this context, however, Δ9-THC has shown biphasic effects, inducing cancer cell growth at low (100–300 nM) [23] and cell death at high (µM) concentrations [24].
While there is ample evidence that cannabinoids and components of the ECS are involved in inhibiting tumor cell proliferation in vitro, little is known about the impacts the ECS has on cells of the TME and consequently on tumor progression. A study by Busch et al. demonstrated that in models of lung adenocarcinoma with different types of mutation (in Kras, p53, or Egfr), the immune cell content varied, suggesting that immune responses and TME landscape of tumors critically depend on tumor cell mutations [25]. As for the ECS, its components are located in immune cells (see Figure 1) besides their expression in tumor cells. Among the few studies that have addressed the ECS in the TME, our group showed, by use of a chemically induced colorectal cancer model, a marked shift in the composition of the immune TME in GPR55 knockout vs. wildtype mice. Knockouts displayed a lower amount of MDSCs which suppress anti-tumor immunity [26], but a higher number of CD4+ and CD8+ cells (which correlate with better prognosis) [27]. Among the other studies, Qiu et al. reported that 2-AG induced the expansion of MDSCs in a model of pancreatic adenocarcinoma with no effect on CD4+ and CD8+ cells [22]. In a model of colon cancer with mice bearing MGL-deficient macrophages, a lower tumor burden was observed in knockouts as compared to wildtypes in a study by Xiang et al. (2018) [28]. Zhu et al. demonstrated that Δ9-THC suppressed host immune reactivity to lung cancer via inhibitory cytokines [29].
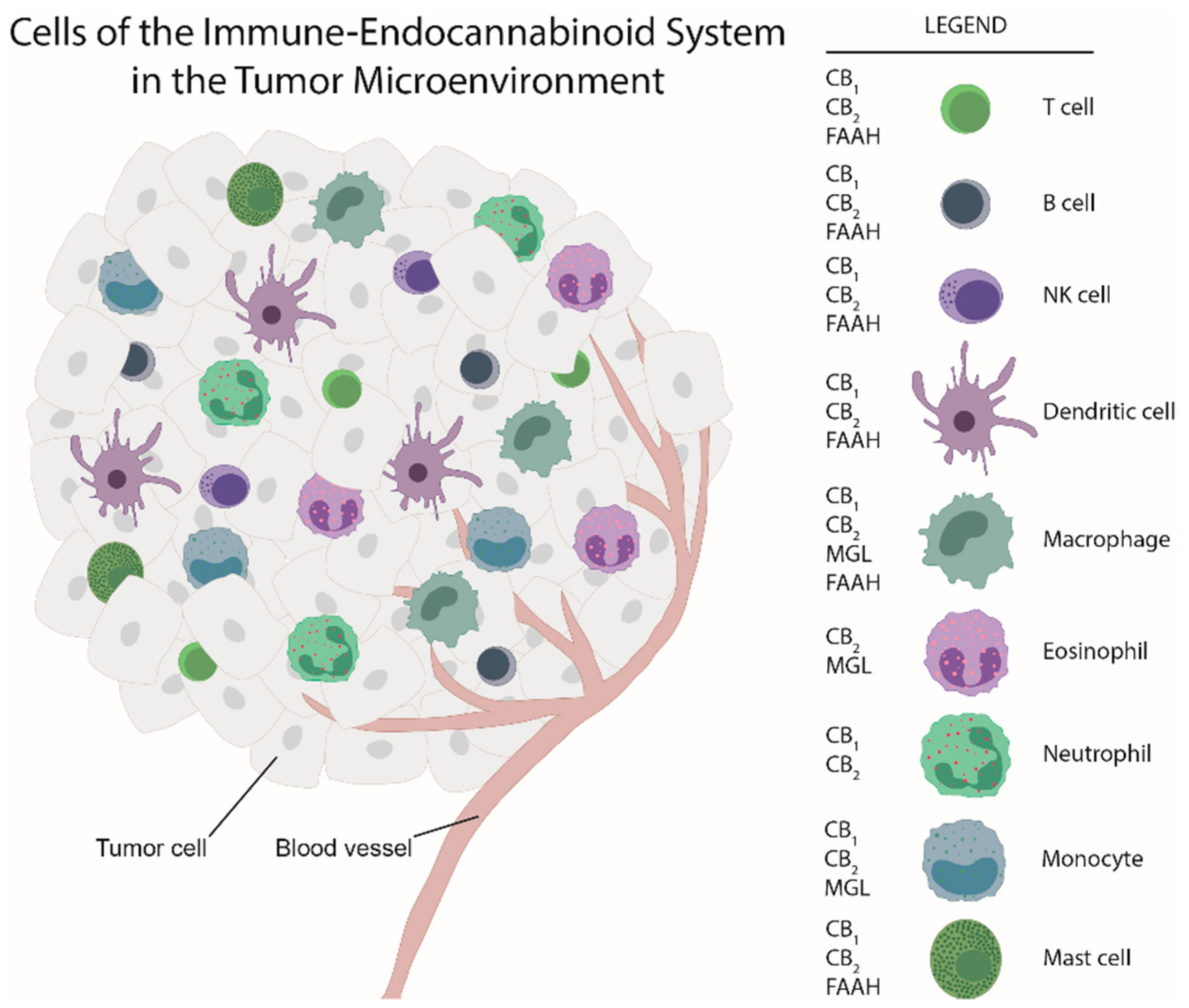
To date, these data suggest that exogenous cannabinoids and ECS components have an influence on immune cells of the TME and that the ECS could be involved in the control of this immune cell network and hence in tumor growth.
3. Conclusions
In vitro studies have demonstrated that the behavior of immune cells is regulated by (endo)cannabinoids and other components of the ECS, indicating that the ECS effectively influences the immune landscape of tumors. This has been now supported by in vivo studies highlighting the importance of macrophages and MDSCs of the TME in the actions of the ECS on tumor growth (e.g., [22][27][28]). ECS components of the TME could be responsible for the fate of tumor growth by working synergistically, independently or in an opposing manner. Knowledge on the role of the ECS in the regulation of the “tumor immune microenvironment” may be important in establishing a more effective anti-neoplastic therapy.
References
- Tomasetti, C.; Li, L.; Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017, 355, 1330–1334.
- Dawson, M.A.; Kouzarides, T.; Huntly, B.J.P. Targeting epigenetic readers in cancer. N. Engl. J. Med. 2012, 367, 647–657.
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146.
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329.
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are mast cells MASTers in cancer? Front. Immunol. 2017, 8.
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306.
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2008, 122, 742–750.
- Benz, A.H.; Renné, C.; Maronde, E.; Koch, M.; Grabiec, U.; Kallendrusch, S.; Rengstl, B.; Newrzela, S.; Hartmann, S.; Hansmann, M.-L.; et al. Expression and functional relevance of cannabinoid receptor 1 in Hodgkin lymphoma. PLoS ONE 2013, 8, e81675.
- Suk, K.-T.; Mederacke, I.; Gwak, G.-Y.; Cho, S.W.; Adeyemi, A.; Friedman, R.; Schwabe, R.F. Opposite roles of cannabinoid receptors 1 and 2 in hepatocarcinogenesis. Gut 2016, 65, 1721–1732.
- Gustafsson, S.B.; Palmqvist, R.; Henriksson, M.L.; Dahlin, A.M.; Edin, S.; Jacobsson, S.O.P.; Öberg, Å.; Fowler, C.J. High Tumour cannabinoid CB1 receptor immunoreactivity negatively impacts disease-specific survival in stage II microsatellite stable colorectal cancer. PLoS ONE 2011, 6, e23003.
- Colangelo, T.; Polcaro, G.; Muccillo, L.; D’Agostino, G.; Rosato, V.; Ziccardi, P.; Lupo, A.; Mazzoccoli, G.; Sabatino, L.; Colantuoni, V. Friend or foe? The tumour microenvironment dilemma in colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 1–18.
- Fraguas-Sanchez, A.I.; Martin-Sabroso, C.; Torres-Suarez, A.I. Insights into the effects of the endocannabinoid system in cancer: A review. Br. J. Pharmacol. 2018, 175, 2566–2580.
- Perez-Gomez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; Garcia-Taboada, E.; Villa-Morales, M.; Moreno, E.; Hamann, S.; Martin-Villar, E.; Flores, J.M.; et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J. Natl. Cancer Inst. 2015, 107, djv077.
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38.
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The endocannabinoid system: A target for cancer treatment. Int. J. Mol. Sci. 2020, 21, 747.
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012.
- Schicho, R. The endocannabinoid system in carcinogenesis. In Mechanisms of Molecular Carcinogenesis–Volume 1; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–10.
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 259–266.
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687.
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952.
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004, 64, 1943–1950.
- Sanchez, C.; Galve-Roperh, I.; Canova, C.; Brachet, P.; Guzman, M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998, 436, 6–10.
- Busch, S.E.; Hanke, M.L.; Kargl, J.; Metz, H.E.; MacPherson, D.; Houghton, A.M. Lung cancer subtypes generate unique immune responses. J. Immunol. 2016, 197, 4493–4503.
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. 2009, 9, 162–174.
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G protein-coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1. Int. J. Cancer 2018, 142, 121–132.
- Xiang, W.; Shi, R.; Kang, X.; Zhang, X.; Chen, P.; Zhang, L.; Hou, A.; Wang, R.; Zhao, Y.; Zhao, K.; et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat. Commun. 2018, 9, 2574.
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 2000, 165, 373–380.
