Plasmonics deals with the free electron vibrations in metal nanostructures, and the interaction of such vibrations with atoms and molecules to create optical nanodevices. A surface plasmon is a collective oscillation of free electrons on the metal surface when the real part of the metal dielectric constant is negative. If the electromagnetic wave is coupled to collective electron oscillations, a surface plasmon wave is induced and propagates along the metal surface, and the electric field in the normal direction to the metal surface is nonradiative and strongly localized at the metal surface. An important phenomenon in plasmonics is the strong spatial localization of electron oscillations at a plasmon resonance frequency. This strong localization leads to a huge increase in the local electric and magnetic fields. Localized surface plasmon resonance (LSPR) is the collective oscillation of free electrons like that of surface plasmon resonance (SPR).
- surface plasmon
- surface-enhanced Raman scattering
- metal-enhanced fluorescence
1. Rhodium- and Platinum-Nanoparticle-Based Optical Sensing
1.1. Rhodium Nanoparticle-Based Optical Sensing
M has weak Raman peaks at 445 cm
−1
−1
−1
10−6
−1
−1
−1
105
1.2. Platinum Nanoparticle-Based Optical Sensing
2. Gold- and Silver-Nanoparticle-Based Optical Sensing
2.1. Gold Nanowire-Based Optical Sensing
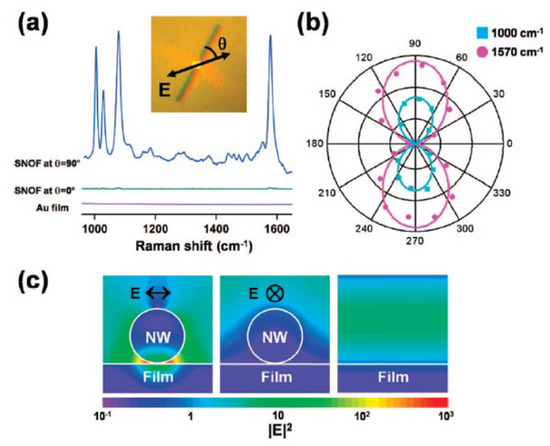
) by a single gold nanowire on a gold film (Au/Au SNOF) [102]. Gold nanowires were synthesized on a sapphire substrate in a horizontal quartz tube furnace system using a vapor transport method.
Figure 2
BT adsorbed on the Au/Au SNOF [102]. The gap-enhanced Raman scattering signal was observed at the gap between the Au nanowire and the Au film when perpendicularly polarized excitation light (
blue
green
BT
2.2. Gold Nanoparticle-Based Optical Sensing
, which was sufficient for single-molecule detection. In these studies, the Raman enhancement was attributed to the plasmon resonance effect of Au nanowires. They reported that the Raman enhancement factors were on the order of
1014
1015
2.3. Silver Nanoparticle-Based Optical Sensing
2.4. Practical Applications of Plasmonic Metal Nanoparticle-Based Optical Sensing
References
- Aslan, K.; Lakowicz, J.R.; Szmacinski, H.; Geddes, C.D. Metal-enhanced fluorescence solution-based sensing platform. J. Fluoresc. 2004, 14, 677–679.
- Darienzo, R.E.; Chen, O.; Sullivan, M.; Mironava, T.; Tannenbaum, R. Au nanoparticles for SERS: Temperature-controlled nanoparticle morphologies and their Raman enhancing properties. Mater. Chem. Phys. 2020, 240, 122143.
- Qayyum, H.; Amin, S.; Ahmed, W.; Mohamed, T.; Rehman, Z.U.; Hussain, S. Laser-based two-step synthesis of Au-Ag alloy nanoparticles and their application for surface-enhanced Raman spectroscopy (SERS) based detection of rhodamine 6G and urea nitrate. J. Mol. Liq. 2022, 365, 120120.
- Kim, K.; Lee, H.S. Effect of Ag and Au nanoparticles on the SERS of 4-aminobenzenethiol assembled on powdered copper. J. Phys. Chem. B 2005, 109, 18929–18934.
- Aslan, K.; Malyn, S.N.; Geddes, C.D. Angular-dependent metal-enhanced fluorescence from silver colloid-deposited films: Opportunity for angular-ratiometric surface assays. Analyst 2007, 132, 1112–1121.
- Aslan, K.; Malyn, S.N.; Geddes, C.D. Metal-enhanced fluorescence from gold surfaces: Angular dependent emission. J. Fluoresc. 2007, 17, 7–13.
- Cabello, G.; Nwoko, K.C.; Marco, J.F.; Sánchez-Arenillas, M.; Méndez-Torres, A.M.; Feldmann, J.; Yáñez, C.; Smith, T.A. Cu@ Au self-assembled nanoparticles as SERS-active substrates for (bio) molecular sensing. J. Alloys Compd. 2019, 791, 184–192.
- de Barros Santos, E.; Sigoli, F.A.; Mazali, I.O. Metallic Cu nanoparticles dispersed into porous glass: A simple green chemistry approach to prepare SERS substrates. Mater. Lett. 2013, 108, 172–175.
- Zhang, Y.; Aslan, K.; Previte, M.J.; Geddes, C.D. Metal-enhanced fluorescence from copper substrates. Appl. Phys. Lett. 2007, 90, 173116.
- Gutiérrez, Y.; Alcaraz de la Osa, R.; Ortiz, D.; Saiz, J.M.; González, F.; Moreno, F. Plasmonics in the ultraviolet with aluminum, gallium, magnesium and rhodium. Appl. Sci. 2018, 8, 64.
- McMahon, J.M.; Schatz, G.C.; Gray, S.K. Plasmonics in the ultraviolet with the poor metals Al, Ga, In, Sn, Tl, Pb, and Bi. Phys. Chem. Chem. Phys. 2013, 15, 5415–5423.
- Tanabe, I.; Shimizu, M.; Kawabata, R.; Katayama, C.; Fukui, K.I. Far-and deep-ultraviolet surface plasmon resonance using Al film for efficient sensing of organic thin overlayer. Sens. Actuators A-Phys. 2020, 301, 111661.
- Knight, M.W.; King, N.S.; Liu, L.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum for plasmonics. ACS Nano 2014, 8, 834–840.
- Gutierrez, Y.; Ortiz, D.; Sanz, J.M.; Saiz, J.M.; Gonzalez, F.; Everitt, H.O.; Moreno, F. How an oxide shell affects the ultraviolet plasmonic behavior of Ga, Mg, and Al nanostructures. Opt. Express 2016, 24, 20621–20631.
- Yang, Y.; Wu, P.C.; Kim, T.H.; Brown, A.S.; Everitt, H.O. Gallium nanoparticle plasmonics. APS March Meet. Abstr. 2010, 55, Y14.00005.
- Gutierrez, Y.; González, F.; Saiz, J.M.; Alcaraz de la Osa, R.; Albella, P.; Ortiz, D.; Everitt, H.O.; Moreno, F. Metals and dielectrics for UV plasmonics. SPIE Proc. Nanophotonics VIII 2020, 11345, 26–34.
- Voet, D.; Gratzer, W.B.; Cox, R.A.; Doty, P. Absorption spectra of nucleotides, polynucleotides, and nucleic acids in the far ultraviolet. Biopolymers 1963, 1, 193–208.
- Cardinal, M.F.; Vander Ende, E.; Hackler, R.A.; McAnally, M.O.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Expanding applications of SERS through versatile nanomaterials engineering. Chem. Soc. Rev. 2017, 46, 3886–3903.
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hot-spot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756.
- Laing, S.; Gracie, K.; Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918.
- Halas, N. Playing with plasmons: Tuning the optical resonant properties of metallic nanoshells. MRS Bull. 2005, 30, 362–367.
- Schlücker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Phys. Plasmas 2014, 53, 4756–4795.
- Kundu, S.; Chen, Y.; Dai, W.; Ma, L.; Sinyukov, A.M.; Liang, H. Enhanced catalytic and SERS activities of size-selective rhodium nanoparticles on DNA scaffolds. J. Mater. Chem. C 2017, 5, 2577–2590.
- Zettsu, N.; McLellan, J.M.; Wiley, B.; Yin, Y.; Li, Z.Y.; Xia, Y. Synthesis, stability, and surface plasmonic properties of rhodium multipods, and their use as substrates for surface-enhanced Raman scattering. Angew. Chem. 2006, 118, 1310–1314.
- Tian, Z.Q.; Ren, B.; Wu, D.Y. Surface-enhanced Raman scattering: From noble to transition metals and from rough surfaces to ordered nanostructures. J. Phys. Chem. B 2002, 106, 9463–9483.
- Hunyadi Murph, S.E.; Coopersmith, K.J. Fabrication of silver-rhodium nanomaterials for chemical sensing applications. In Nanocomposites VI: Nanoscience and Nanotechnology in Advanced Composites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–104.
- Sangeetha, K.; Sankar, S.S.; Karthick, K.; Anantharaj, S.; Ede, S.R.; Wilson, S.; Kundu, S. Synthesis of ultra-small Rh nanoparticles congregated over DNA for catalysis and SERS applications. Colloids Surf. B Biointerfaces 2019, 173, 249–257.
- Kumaravel, S.; Karthick, K.; Sankar, S.S.; Karmakar, A.; Kundu, S. Engineered Rh Nano-networks on DNA for SERS Applications. J. Nanotechnol. Nanomater. 2020, 1, 57–64.
- Zhang, Y.; Geddes, C.D. Metal-enhanced fluorescence from thermally stable rhodium nanodeposits. J. Mater. Chem. 2010, 20, 8600–8606.
- Rodrigues, M.P.D.S.; Dourado, A.H.; Cutolo, L.D.O.; Parreira, L.S.; Alves, T.V.; Slater, T.J.; Haigh, S.J.; Camargo, P.H.C.; Cordoba de Torresi, S.I. Gold-rhodium nanoflowers for the plasmon-enhanced hydrogen evolution reaction under visible light. ACS Catal. 2021, 11, 13543–13555.
- Akbay, N.; Mahdavi, F.; Lakowicz, J.R.; Ray, K. Metal-enhanced intrinsic fluorescence of nucleic acids using platinum nanostructured substrates. Chem. Phys. Lett. 2012, 548, 45–50.
- Chu, C.S.; Sung, T.W.; Lo, Y.L. Enhanced optical oxygen sensing property based on Pt (II) complex and metal-coated silica nanoparticles embedded in sol–gel matrix. Sens. Actuators B-Chem. 2013, 1852, 287–292.
- Bigall, N.C.; Hartling, T.; Klose, M.; Simon, P.; Eng, L.M.; Eychmuller, A. Monodisperse platinum nanospheres with adjustable diameters from 10 to 100 nm: Synthesis and distinct optical properties. Nano Lett. 2008, 8, 4588–4592.
- Mafune, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T. Formation of stable platinum nanoparticles by laser ablation in water. J. Phys. Chem. B 2003, 107, 4218–4223.
- Cueto, M.; Piedrahita, M.; Caro, C.; Martinez-Haya, B.; Sanz, M.; Oujja, M.; Castillejo, M. Platinum nanoparticles as photoactive substrates for mass spectrometry and spectroscopy sensors. J. Phys. Chem. C 2014, 118, 11432–11439.
- Kim, K.; Lee, H.B.; Choi, J.Y.; Kim, K.L.; Shin, K.S. Surface-enhanced Raman scattering of 4-aminobenzenethiol in nanogaps between a planar Ag substrate and Pt nanoparticles. J. Phys. Chem. C 2011, 115, 13223–13231.
- Kim, K.; Kim, K.L.; Lee, H.B.; Shin, K.S. Surface-enhanced Raman scattering on aggregates of platinum nanoparticles with definite size. J. Phys. Chem. C 2010, 114, 18679–18685.
- Yang, Y.; Huang, Z.; Li, D.; Nogami, M. Solvothermal synthesis of platinum nanoparticles and their SERS properties. In Proceedings of the 5th International Symposium on Advanced Optical Manufacturing and Testing Technologies: Optoelectronic Materials and Devices for Detector, Imager, Display, and Energy Conversion Technology, Dalian, China, 26–29 April 2010; Volume 7658, p. 76580H.
- Cui, L.; Wang, A.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Shaping and shelling Pt and Pd nanoparticles for ultraviolet laser excited surface-enhanced Raman scattering. J. Phys. Chem. C 2008, 112, 17618–17624.
- Abdelsalam, M.E.; Mahajan, S.; Bartlett, P.N.; Baumberg, J.J.; Russell, A.E. SERS at structured palladium and platinum surfaces. J. Am. Chem. Soc. 2007, 23, 7399–7406.
- Kämmer, E.; Dorfer, T.; Csaki, A.; Schumacher, W.; Da Costa Filho, P.A.; Tarcea, N.; Fritzsche, W.; Rösch, P.; Schmitt, M.; Popp, J. Evaluation of colloids and activation agents for determination of melamine using UV–SERS. J. Phys. Chem. C 2012, 116, 6083–6091.
- Tran, M.; Whale, A.; Padalkar, S. Exploring the efficacy of platinum and palladium nanostructures for organic molecule detection via Raman spectroscopy. Sensors 2018, 18, 147.
- Fan, Y.; Girard, A.; Waals, M.; Salzemann, C.; Courty, A. Ag@Pt core-shell nanoparticles for plasmonic catalysis. ACS Appl. Nano Mater. 2023, 6, 1193–1202.
- Pang, P.; Teng, X.; Chen, M.; Zhang, Y.; Wang, H.; Yang, C.; Yang, W.; Barrow, C.J. Ultrasensitive enzyme-free electrochemical immunosensor for microcystin-LR using molybdenum disulfide/gold nanoclusters nanocomposites as platform and Au@ Pt core-shell nanoparticles as signal enhancer. Sens. Actuators B Chem. 2018, 266, 400–407.
- Proniewicz, E.; Gralec, B.; Ozaki, Y. Homogeneous Pt nanostructures surface functionalized with phenylboronic acid phosphonic acid derivatives as potential biochemical nanosensors and drugs: SERS and TERS studies. J. Biomed. Mater. Res. Part B 2023, 111.
- Lin, S.; Ze, H.; Zhang, X.G.; Zhang, Y.J.; Song, J.; Zhang, H.; Zhong, H.L.; Yang, Z.L.; Yang, C.; Li, J.F.; et al. Direct and simultaneous identification of multiple mitochondrial reactive oxygen species in living cells using a SERS borrowing strategy. Angew. Chem. Int. Ed. 2015, 61, e202203511.
- Yoon, I.; Kang, T.; Choi, W.; Kim, J.; Yoo, Y.; Joo, S.W.; Park, Q.H.; Ihee, H.; Kim, B. Single Nanowire on a Film as an Efficient SERS-Active Platform. J. Am. Chem. Soc. 2009, 131, 758–762.
- Ranjan, M.; Facsko, S. Anisotropic surface enhanced Raman scattering in nanoparticle and nanowire arrays. Phys. Plasmas 2012, 23, 485307.
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460.
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106.
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670.
- Kneipp, K.; Haka, A.S.; Kneipp, H.; Badizadegan, K.; Yoshizawa, N.; Boone, C.; Shafer-Peltier, K.E.; Motz, J.T.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman spectroscopy in single living cells using gold nanoparticles. Appl. Spectrosc. 2002, 56, 150–154.
- Qian, X.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90.
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712.
- He, R.X.; Liang, R.; Peng, P.; Zhou, Y.N. Effect of the size of silver nanoparticles on SERS signal enhancement. J. Nanopart. Res. 2017, 19, 267.
- Lee, S.J.; Morrill, A.R.; Moskovits, M. Hot spots in silver nanowire bundles for surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2006, 128, 2200–2201.
- Fan, M.; Brolo, A.G. Silver nanoparticles self assembly as SERS substrates with near single molecule detection limit. Phys. Chem. Chem. Phys. 2009, 11, 7381–7389.
- Gopal, J.; Abdelhamid, H.N.; Huang, J.H.; Wu, H.F. Nondestructive detection of the freshness of fruits and vegetables using gold and silver nanoparticle mediated graphene enhanced Raman spectroscopy. Sens. Actuators B-Chem. 2016, 224, 413–424.
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-enhanced Raman spectroscopy applied to food safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380.
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of nanotechnology in food industry: Challenges in processing, packaging, and food safety. Glob. Chall. 2023, 7, 2200209.
- Terry, L.R.; Sanders, S.; Potoff, R.H.; Kruel, J.W.; Jain, M.; Guo, H. Applications of surface-enhanced Raman spectroscopy in environmental detection. Anal. Sci. Adv. 2022, 3, 113–145.
- Hail, C.U.; Michel, A.K.U.; Poulikakos, D.; Eghlidi, H. Optical metasurfaces: Evolving from passive to adaptive. Adv. Opt. Mater. 2019, 7, 1801786.
- Meinzer, N.; Barnes, W.L.; Hooper, I.R. Plasmonic meta-atoms and metasurfaces. Nat. Photonics 2014, 8, 889–898.
- Gwo, S.; Chen, H.Y.; Lin, M.H.; Sun, L.; Li, X. Nanomanipulation and controlled self-assembly of metal nanoparticles and nanocrystals for plasmonics. Chem. Soc. Rev. 2016, 45, 5672–5716.
- Gwo, S.; Wang, C.Y.; Chen, H.Y.; Lin, M.H.; Sun, L.; Li, X.; Chen, W.L.; Chang, Y.M.; Ahn, H. Plasmonic metasurfaces for nonlinear optics and quantitative SERS. ACS Photonics 2016, 22, 1371–1384.
 Encyclopedia
Encyclopedia
