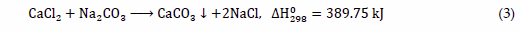Technologies for the management of various types of waste and the production of useful products from them are currently widely studied. Both carbon dioxide and calcium-rich waste from various production processes are problematic wastes that can be used to produce calcium carbonate. Therefore, the purpose of this paper is to provide an overview about the state of the development of processes that use these two wastes to obtain a valuable CaCO3 powder.
- Calcium Carbonate
- Carbon capture and storage
- carbon capture and utilization
1. Introduction
Carbon capture and storage (CCS) and carbon capture and utilization (CCU) technologies are the most popular processes to reduce CO2 emissions [7,8,9][1][2][3] and play an important role in meeting the global targets specified at COP25 [10][4]. In the case of both technologies, three main CO2 capture systems depending on the type of combustion process can be distinguished, post-combustion, pre-combustion, and oxyfuel combustion [6,8,9][5][2][3]. The choice of capture technology depends on the type of plant, i.e., the composition of the exhaust gas [11,12][6][7]. Post-combustion technology is the simplest to implement and is mainly based on chemical absorption [9,13,14][3][8][9]. Thus, this option is usually used as a modification to existing power plants. However, due to the low CO2 content in flue gases (4% in the case of natural gas combustion, 7–14% in the case of coal combustion), it is relatively expensive to obtain a gas stream with more than 95% CO2 using this technology. This is due to the fact that low CO2 concentration negatively affects the capture efficiency [15][10].
CCS technology is applicable to large CO2 point emission sources and involves the capture of waste CO2 from plants, including power plants or cement factories and then its transport to the storage site [11,16][6][11]. Most often, waste CO2 is stored in geological formations. The advantage of CCS technology is its high CO2 capture efficiency, which is usually above 80% [15][10]. However, large-scale deployment of CCS technology is not widespread so far due to both economic and technical barriers. First of all, the main obstacle is the unprofitability of such technology, as it requires a large financial investment [17][12]. Moreover, in some countries such as Norway, Great Britain, India, or Brazil, the geological possibilities for CO2 storage are very limited, which increases the costs of transport and injection, making the CCS solution an unrealistic option [18][13].
In order for CO2 capture to be a cost-effective process, either more efficient absorbents than commonly used should be applied, or alternative technological strategies should be implemented. In recent years, many alternatives to conventional CCS technologies, such as CCU, have been proposed [19,20,21][14][15][16]. Such technologies allow for reuse of captured CO2, which may partially offset the total cost of carbon capture and storage [16][11]. CCU technology includes CO2 absorption from anthropogenic emissions and its use as a substrate for the synthesis of valuable products such as concrete, plastics, fillers, or reagents for chemical synthesis [4,7,22,23,24][17][1][18][19][20]. One of the processes in which waste carbon dioxide from various sources can be applied as a substrate is carbon mineralization. An overview of mineral carbonation and its market potential has recently been presented [25][21] and an economic analysis of the various methods of CO2 utilization has been prepared by Hepburn et al. [26][22]. They show that mineral carbonation is one of the methods that comes closest to large-scale commercialization due to thermodynamically favored and economic considerations. Furthermore, it is possible to use numerous industrial wastes as other substrates when the product of the mineral carbonation is calcium carbonate. Such solution ensures effective recycling of both gaseous CO2 and liquid industrial waste, and in addition obtained final product can be applied in many industries [25][21].
2. CaCO3 Precipitation Methods
2.1. Carbonation Method
Carbonation is the main method used for the production of calcium carbonate on an industrial scale. This process involves the introduction of gaseous carbon dioxide into the reaction mixture being an aqueous solution containing calcium ions. Calcium hydroxide suspension (gas-suspension system) is used as the source of Ca2+ ions and in this case the alkaline environment causes CO2 to be easily absorbed [27][23]. Equation (1) provides the overall reaction:
The alternative is a gas-liquid reaction with a Ca2+ ion source in the form of an aqueous calcium salt solution such as CaCl2 and with the presence of absorption promoter such as amines or ammonia. In the presence of ammonia, the overall reaction presented in Equation (2) occurs in the solution:
Generation of supersaturation in solution, and consequently induction of calcium carbonate crystals nucleation and growth is possible due to CO2 absorption, which rate is the limiting factor. Ammonia hydrolysis results in the formation of hydroxyl ions. Bicarbonate ions are formed rapidly by the direct reaction between CO2 and OH− ions, after which they are transformed to carbonate ions. The last stage is the reaction between Ca2+ and CO32−, whereby CaCO3 is precipitated [28][24]. Changes in the composition of the aqueous phase cause a change in the solubility of carbon dioxide, which affects the driving force of absorption [29][25]. The rate of CO2 absorption in aqueous solutions, in turn, has an impact on the rate of chemical reaction between carbonate and calcium ions, and depends on parameters such as mass transfer coefficient, equilibrium and actual CO2 solubility, and contact liquid gas. When the absorption of carbon dioxide is accelerated, the rate of precipitation increases as well [29][25]. Temperature is an important parameter influencing the course of the calcium carbonate formation. The solubility of CO2 in solution is described by Henry’s law and the increase in temperature results in a decrease in CO2 solubility, and thus in a decrease in the rate of CO2 absorption [30][26]. On the other hand, the reactions of CaCO3 formation with the use of gaseous CO2 are exothermic, i.e., with the release of heat. Therefore, most of the research is conducted under moderate temperature conditions, which favors the CO2 transfer from the gas phase to the liquid phase.
2.2. Liquid-Liquid Method
Another method of calcium carbonate production used in industry is the liquid-liquid process in which aqueous solutions containing calcium and carbonate ions are mixed [31][27]. It is often used in laboratory tests due to the ease of controlling process variables [32][28]. In the literature, a lot of studies can be found in which aqueous solutions of CaCl2 and Na2CO3 [33][29], as well as Ca(OH)2 and H2CO3 [34][30] are used. The source of carbonate ions can also be (NH4)2CO3, while calcium acetate as the source of Ca2+ ions [35][31] may be applied. An example of a general reaction can be written as Equation (3):
3. Conclusions
Nowadays, a lot of attention is paid to the issue of the increasing amount of produced industrial waste, which is a serious environmental hazard. Numerous attempts are made to develop and implement technologies aimed at both disposal and reuse of this waste. Carbon dioxide emitted during the combustion of fuels or released during the manufacture of many products is indicated as one of the main greenhouse gas that contributes to rapid global warming. Another group of nuisance pollutants is calcium-rich wastes, such as distillation liquid from the Solvay process, metallurgical slag from steel production, cement industry waste, gypsum waste, ash from paper sludge and oil shale ash. The alkalinity of individual liquid waste and the liquid after leaching of solid waste allows them to be used to capture carbon dioxide from flue gas. Therefore, an interesting idea for the management of CO2 and Ca-rich waste is to develop a technology for producing calcium carbonate via carbonation route. Such technologies help reduce harmful emissions while producing a valuable product that can be sold and used in many industries, such as paper, rubber, pharmaceuticals, and many others.
Therefore, an important criterion in the evaluation of these technologies is the possibility of producing CaCO3 meeting numerous criteria, such as high purity, adequate humidity, particle size, and shape, or polymorphic form. In addition, for the technology of calcium carbonate precipitation from waste liquids and CO2 to be successfully implemented in production, this process must be characterized by low electricity demand. In general, the technology must be economically viable, so that the benefit from the sale of precipitated CaCO3 should be more profitable higher than the cost of its production. Therefore, most of the technologies proposed and described in this work are carried out at room temperature and atmospheric pressure, so as not to generate additional costs associated with heating or maintaining the desired pressure of the reaction system. However, there are many other obstacles, such as the necessary reagent regeneration processes, which are expensive, or the generation of other waste streams for which further utilization processes should be implemented. An important issue is also the fact that most potential Ca-rich waste is in solid form, which requires the extraction of calcium ions into the solution. Therefore, the efficiency of the Ca ion leaching process is another important factor influencing the profitability of the designed process. In addition, the implementation of a given waste management technology may also be determined by political factors, when legal regulations of a given country force entrepreneurs to reduce emissions and pollutants.
References
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium carbonate precipitation for CO2 storage and utilization: A review of the carbonate crystallization and polymorphism. Front. Energy Res. 2017, 5, 1–12.
- Blomen, E.; Hendriks, C.; Neele, F. Capture technologies: Improvements and promising developments. Energy Procedia 2009, 1, 1505–1512.
- Robles, J.O.; Almaraz, S.D.-L.; Azzaro-Pantel, C. Hydrogen Supply Chain Design: Key Technological Components and Sustainable Assessment. In Hydrogen Supply Chains. Design, Deployment and Operation; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–79.
- COP25 Paris Agreement, European Commission. Available online: https://unfccc.int/cop25 (accessed on 16 November 2020).
- Loo, L.; Konist, A.; Neshumayev, D.; Pihu, T.; Maaten, B.; Siirde, A. Ash and flue gas from oil shale oxy-fuel circulating fluidized bed combustion. Energies 2018, 11, 1218.
- Thambimuthu, K.; Soltanieh, M.; Abanades, J.C. Capture of CO2. In Carbon Dioxide Capture and Storage; Metz, B., Davidson, O., de Coninck, H., Loos, M., Meyer, L., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 105–178.
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692.
- Pellegrini, G.; Strube, R.; Manfrida, G. Comparative study of chemical absorbents in postcombustion CO2 capture. Energy 2010, 35, 851–857.
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720.
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176.
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102.
- Khoo, H.H.; Sharratt, P.N.; Bu, J.; Yeo, T.Y.; Borgna, A.; Highfield, J.G.; Björklöf, T.G.; Zevenhoven, R. Carbon capture and mineralization in singapore: Preliminary environmental impacts and costs via LCA. Ind. Eng. Chem. Res. 2011, 50, 11350–11357.
- Sha, F.; Zhu, N.; Bai, Y.; Li, Q.; Guo, B.; Zhao, T.; Zhang, F.; Zhang, J. Controllable Synthesis of Various CaCO3 Morphologies Based on a CCUS Idea. ACS Sustain. Chem. Eng. 2016, 4, 3032–3044.
- Zevenhoven, R.; Legendre, D.; Said, A.; Järvinen, M. Carbon dioxide dissolution and ammonia losses in bubble columns for precipitated calcium carbonate (PCC) production. Energy 2019, 175, 1121–1129.
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045.
- Zdeb, J.; Howaniec, N.; Smoliński, A. Utilization of carbon dioxide in coal gasification—An experimental study. Energies 2019, 12, 140.
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions. J. CO2 Util. 2017, 22, 346–354.
- Abidin, V.; Bouallou, C.; Clodic, D. Valorization of CO2 emissions into ethanol by an innovative process. Chem. Eng. Trans. 2011, 25, 1–6.
- Mendes, L.; De Medeiros, J.L.; Alves, R.M.B.; Araújo, O.Q.F. Production of methanol and organic carbonates for chemical sequestration of CO2 from an NGCC power plant. Clean Technol. Environ. Policy 2014, 16, 1095–1105.
- Woodall, C.M.; McQueen, N.; Pilorgé, H.; Wilcox, J. Utilization of mineral carbonation products: Current state and potential. Greenh. Gases Sci. Technol. 2019, 9, 1096–1113.
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97.
- Ding, Y.; Liu, Y.; Ren, Y.; Yan, H.; Wang, M.; Wang, D.; Lu, X.Y.; Wang, B.; Fan, T.; Guo, H. Controllable synthesis of all the anhydrous CaCO3 polymorphs with various morphologies in CaCl2-NH3-CO2 aqueous system. Powder Technol. 2018, 333, 410–420.
- Ding, L.; Wu, B.; Luo, P. Preparation of CaCO3 nanoparticles in a surface-aerated tank stirred by a long-short blades agitator. Powder Technol. 2018, 333, 339–346.
- Wachi, S.; Jones, A.G. Mass Transfer with Chemical Precipitation Reaction. Chem. Eng. Sci. 1991, 46, 1027–1033.
- Green, D.W.; Perry, R.H. (Eds.) Perry’s Chemical Engineers’ Handbook, 8th ed.; The McGraw-Hill Companies: New York, NY, USA, 2008.
- Mori, Y.; Enomae, T.; Isogai, A. Preparation of pure vaterite by simple mechanical mixing of two aqueous salt solutions. Mater. Sci. Eng. C 2009, 29, 1409–1414.
- Ukrainczyk, M.; Kontrec, J.; Babić-Ivančić, V.; Brečević, L.; Kralj, D. Experimental design approach to calcium carbonate precipitation in a semicontinuous process. Powder Technol. 2007, 171, 192–199.
- Kitamura, M. Controlling factor of polymorphism in crystallization process. J. Cryst. Growth 2002, 237–239, 2205–2214.
- Kralj, D.; Kontrec, J.; Brečević, L.; Falini, G.; Nöthig-Laslo, V. Effect of Inorganic Anions on the Morphology and Structure of Magnesium Calcite. Chem. A Eur. J. 2004, 10, 1647–1656.
- Wen, Y.; Xiang, L.; Jin, Y. Synthesis of plate-like calcium carbonate via carbonation route. Mater. Lett. 2003, 57, 2565–2571.